Table of Contents
Cells were designed and successfully operated on a laboratory scale for electrowinning high-purity neodymium, praseodymium, and didymium, as well as the higher melting-point metals, gadolinium, dysprosium, and yttrium. The elements were prepared from their oxides, dissolved in fluoride melts, and deposited as liquid metals on a tungsten cathode. Similarly, alloys of these metals and samarium, with selected ferrous metals, were prepared by electrode-positing the rare-earth metals on consumable cathodes fabricated from the ferrous metals.
A cell was designed for winning cerium and lanthanum metals from their oxides in pound quantities by a continuous process. The liquid metals were tapped from the cell and cast into ingots.
Preparation of high-purity rare-earth metals and their alloys by electrowinning is part of the Bureau of Mines program for recovering reactive metals from oxides or naturally occurring compounds. Studies are directed toward winning the metal products in the liquid state rather than preparing high-surface-area dendritic products, thus minimizing contamination and facilitating metal-electrolyte separation. This report summarizes the techniques developed for preparing rare-earth metals and their alloys by electrolysis of the oxides in fluoride melts.
The rare-earth oxides are very stable, as evidenced by the average free energy of formation, which is greater than -110 kcal per gram atom of oxygen at 1,500° K. This value can be compared with values for aluminum oxide of -96 kcal and magnesium oxide at about -102 kcal. In the reduction of rare-earth oxides by carbon, thermodynamic calculations and metallurgical practice show that formation of the carbides is favored instead of reduction to metal.
Methods for preparing the rare-earth metals, therefore, are limited to electrowinning procedures, or to reducing the oxides or salts with energy-expensive metals, such as calcium.
Most of the earlier work on the electrowinning of rare-earth metals was done on a laboratory scale utilizing chloride salts, and has been reviewed in another paper. Cerium, lanthanum, and misch metal are now produced commercially by electrolysis of anhydrous chlorides in alkali or alkaline-earth chloride melts at relatively low temperatures. Many of the rare-earth metals, however, have melting points above 1,000° C. Electrolytic preparation of metals in the liquid state requires electrolytes with special properties not met by chloride electrolytes. These properties are low vapor pressure at high temperatures, ability to readily dissolve rare-earth oxide, and a cation that will not be coreduced with the rare-earth ion. With these factors in mind, the electrolytes chosen for winning neodymium, praseodymium, samarium, gadolinium, yttrium, dysprosium, and didymium (a cerium-free mixture of the light rare-earth metals) contained 50 mole-percent of LiF and 50 mole-percent of the respective rare-earth fluorides. The cerium and lanthanum electrolytes, however, were ternary mixtures that also included barium fluoride. The solubility of the rare-earth oxides in fluoride electrolytes was determined to be from 2 to 4 percent by a method described previously.
It was believed that problems, such as carbon contamination and reaction of the higher melting rare-earth metals (>1,300° C) with the electrolyte, could be minimized by electrolytically preparing these metals as alloys. Certain alloys have potential uses in industry and in nuclear technology, as well as being a source material for preparation of the reactive metals. Examples of the uses of these alloys include rare-earth-cobalt alloys for permanent magnets and rare-earth-chromium alloys for resistance of high-temperature oxidation.
Cell Design and Operation
Studies on the direct electrowinning of the rare-earth elements and preparation of their alloys resulted in the design and development of three electrolytic cells. Two cells, each approximately 6 inches in diameter, were used to determine operating parameters (such as bath composition, oxide feed rate, and electrolysis temperature) for preparation of high-purity metallic products in a batch-type operation. The third cell, approximately 12 inches in diameter, was used to determine optimum conditions for continuous cell operation and removal of molten cerium and lanthanum metals. In the 6- inch-diameter cells the liquid metal or alloy that formed at the cathode was collected in the form of nodules in the lower portion of the cell. One of the 6-inch-diameter cells was used for investigations of the cerium-group metals and on alloys melting below 1,300° C; the other cell was operated at temperatures up to 1,700° C for preparing liquid yttrium-group metals.
Certain features were common to each of the cells:
- Each was enclosed in an inert-atmosphere chamber to prevent reaction of the liquid rare-earth metal or alloy with air and moisture. The chamber was described in a previous publication.
- Each cell was internally heated. The necessary heat in the reaction zone of the 12-inch cell was provided solely by the electrolysis current, and the heating in the 6-inch cells was supplemented by imposition of alternating current.
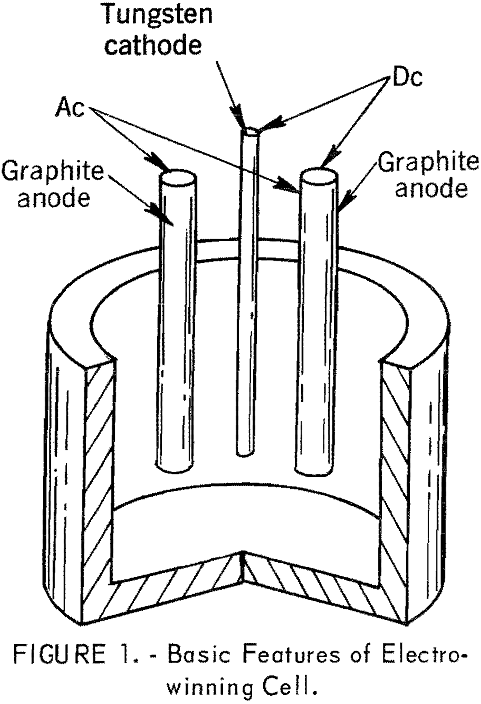
- The anodes and the crucible for containing the molten electrolyte were fabricated from AGX-grade graphite stock. For electrowinning individual rare-earth metals, the cathode was tungsten or molybdenum; for preparing alloy, the cathode was a consumable rod of the material that was to be alloyed with the rare-earth metal.
- The crucible was placed inside a shell fabricated from heat-insulating mullite brick and alumina. The removable top of the shell was also made of mullite brick and had a graphite facing to protect the brick from erosion by the fluoride vapors.
Electrowinning Low-Melting Rare-Earth Metals
The 6-inch-diameter cell for electrowinning neodymium, praseodymium, and didymium metals is shown in figure 1. To maintain the requisite bath temperature for electrolysis, more heat was required than could be supplied by direct current power. The cell, therefore, was provided with auxiliary heating by the imposition of alternating current on the anodes.
Graphite contamination of the rare-earth metal was minimized by collecting the liquid metal on a frozen electrolyte skull. The skull was maintained by carefully controlling the temperature of the electrolyte and cooling the bottom of the crucible to balance the heat input when necessary. Temperature measurements of the bath in the cathode zone were made intermittently with an immersion thermocouple. Another thermocouple, located in the crucible bottom, was used to measure the temperature of the metal collection zone.
Before starting electrowinning an inert atmosphere was obtained in the cell chamber by pumping and backfilling with helium. The fluoride electrolyte was charged to the cell, and meltdown was started at the top of the electrolyte container by striking an arc to the cathode with a tungsten probe. After a molten pool of electrolyte was obtained, the probe was removed, alternating current was applied to the anodes, and heating continued until the required cell temperature was attained. Electrolysis was then started and the rare-earth oxide was intermittently fed to the cell. After the electrowinning experiment was completed, the electrolyte was allowed to freeze and the metal was subsequently recovered.
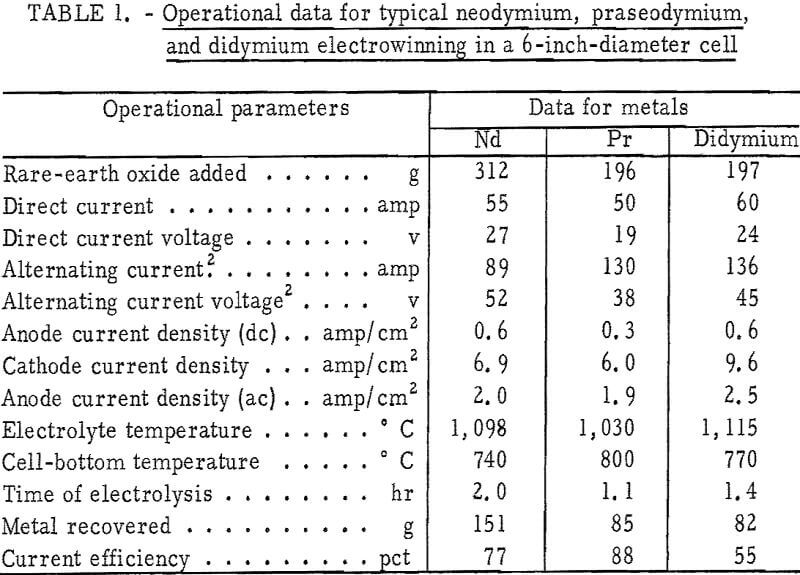
Operational data for typical neodymium, praseodymium, and didymium electrowinning experiments are given in table 1. Analysis of the atmosphere in the cell chamber during electrolysis showed that a gas mixture of carbon oxides, with trace amounts of fluorocarbons, was evolved from the anodes. Good coalescence of the metal products was obtained, and nodules weighing up to 120 grams were recovered. Current efficiencies up to 88 percent were attained.
Preparation of Alloys by Direct Electrolysis
The cell for preparing rare-earth alloys by electrolysis had the same design as the cell for preparing neodymium, praseodymium, and didymium metals, except that the cathode consisted of a consumable rod of iron, nickel, chromium, or cobalt. The rare-earth metal was deposited on the cathode to form an alloy with the cathode metal. Electrolyte compositions were the same as those used in electrowinning the individual metals. The cell was operated at a temperature below the melting point of the ferrous-metal cathode and above the melting point of the eutectic formed between the rare-earth metal and the ferrous metal. As the alloy formed on the cathode and dripped off, the cathode was penciled down, and more rod was lowered into the electrolyte.
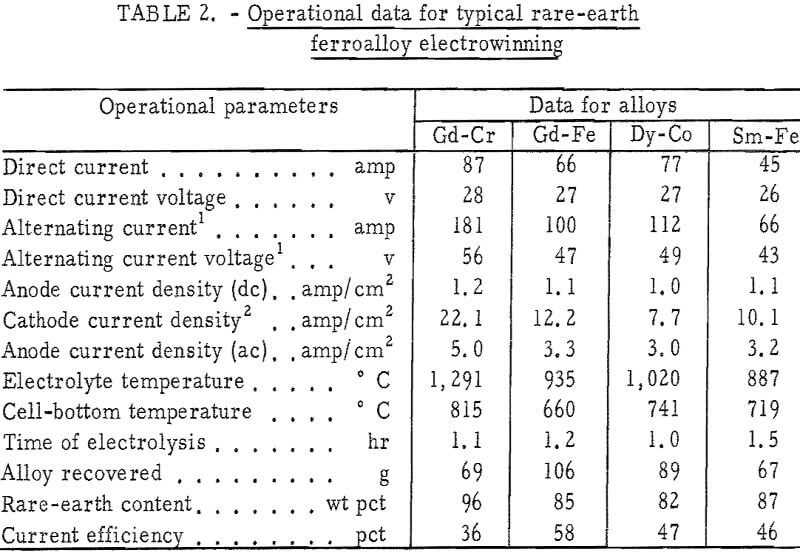
Table 2 gives operational data for electrowinning typical alloys of gadolinium with iron and chromium, samarium with iron, and dysprosium with cobalt. Current efficiencies, based on trivalent reactions, ranged from 36 to 58 percent. The major off gases were carbon oxides, with carbon monoxide predominating at the higher cell-operating temperatures. Alloy nodules weighing 2 to 100 grams were easily separated from the frozen electrolyte. Their composition was temperature dependent and was generally in the vicinity of the eutectic composition. In table 3, the compositions of electrowon alloys are compared with the value expected from the phase diagrams.
Of particular interest was the electrowinning of a samarium-iron alloy. Previous investigators have been unable to prepare samarium either by electrolysis or by metallothermic reduction of its halides because of the stability of the divalent ion. By producing a samarium-iron alloy, back reaction of samarium with its fluoride was inhibited, and alloy nodules weighing as much as 15 grams and containing 87 weight-percent samarium were obtained.
Electrowinning High-Melting Rare-Earth Metals
Certain problems encountered in the low-temperature operation were greatly magnified when attempts were made to electrowin the yttrium-group elements in liquid state at temperatures as high as 1,700° C. The main problem areas were (1) the energy required to maintain electrolyte temperatures above the melting point of the rare-earth metals, (2) the control of the temperature in the metal collection zone, and (3) the materials of construction capable of with-standing both the elevated temperatures and the corrosive nature of the salts. The essential features of the high-temperature cell are shown in figure 2.
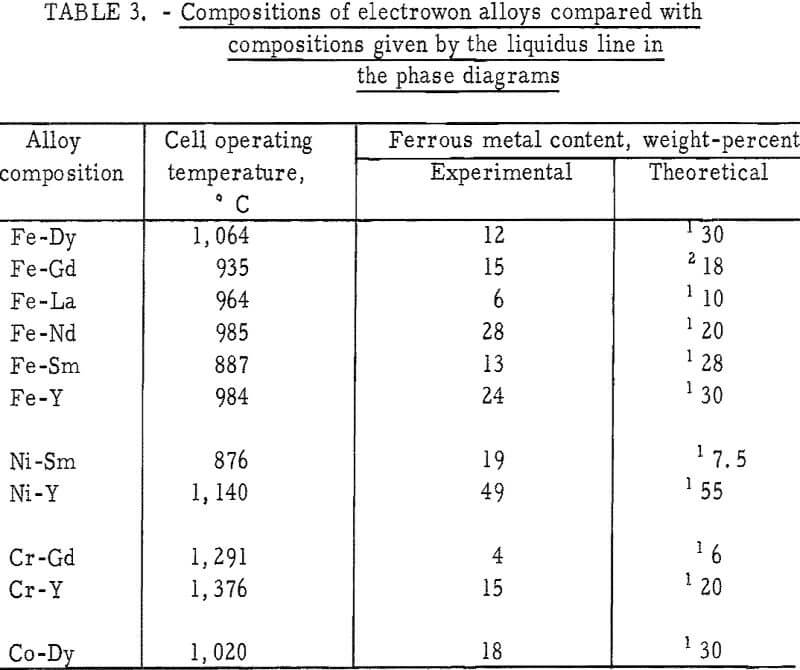
The necessary heat in the reaction zone of the cell was obtained by applying alternating current to two graphite anodes which supplied approximately three-fourths of the total power. Considerably more energy was required to maintain the temperature in the high-temperature cell than in the cell for winning cerium-group metals. For example, the power requirement for electrowinning praseodymium was 6 kw, whereas 14 kw was used in preparing gadolinium. The anode reaction, the formation of CO, appears to be an important rate-controlling factor in the operation of the cell; therefore, the graphite anodes were fluted to provide as large a surface area as practicable. This larger surface area also permitted shallower immersion of the anodes, thereby facilitating escape of the carbon oxides from the bath.
Experience indicated that a relationship exists between the temperature of the metal collection zone and the recovery and purity of the product. To minimize back reaction with the electrolyte and contamination of the metal, nodules were collected on a frozen electrolyte skull in the lower portion of the cell, which was maintained at approximately 500° C below the melting point of the reactive metal. The conditions for operating the high-temperature cell were obtained by internally heating the electrolyte and, at the same time,
cooling the bottom of the crucible by passing helium or air through a copper coil placed below the crucible.
Materials necessary for construction and operation of the high-temperature cell were taxed nearly to their limits, and only by using a combination of materials could a satisfactory cell be designed. The graphite crucible was placed inside a shell fabricated from layers of various insulating materials. The inner wall of the shell was made from fused alumina. Castable alumina, 2 inches thick, was then placed around the inner wall. Finally, there was an outer wall, 4 inches thick, made from mullite brick.
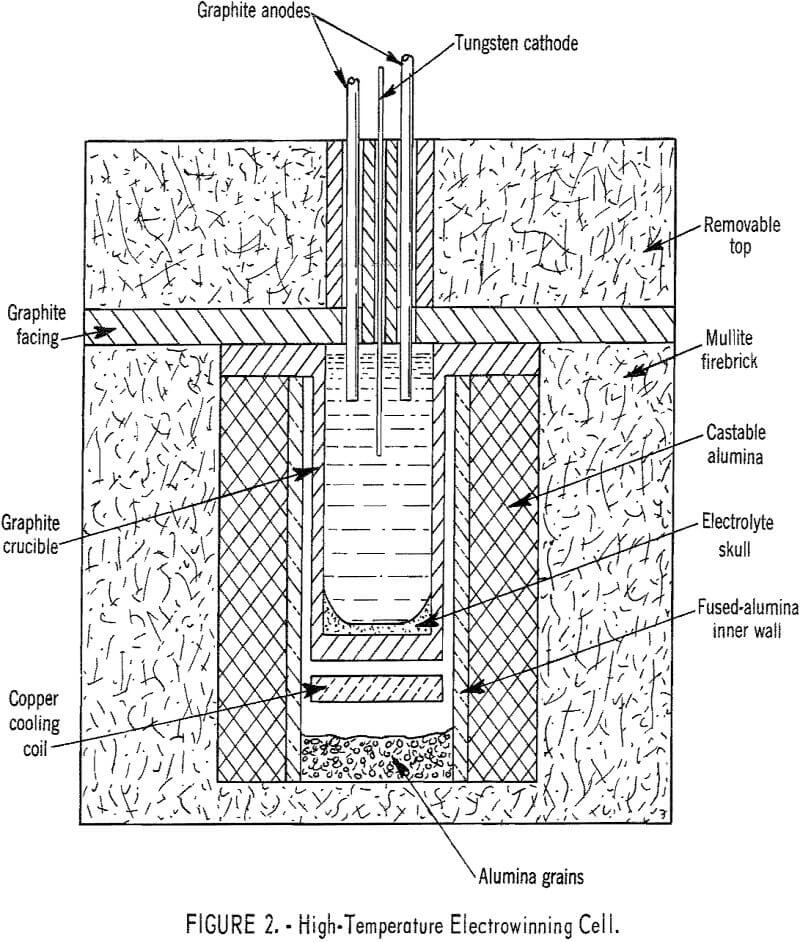
Operation of the high-temperature cell was demonstrated by preparing liquid gadolinium, dysprosium, and yttrium metals in the temperature range of 1,370° to 1,700° C. Metal nodules weighing from 1 to 20 grams were recovered. Operational data for a typical gadolinium experiment are given in table 4. In a typical yttrium electrowinning experiment the temperature of the cathode zone was maintained at 1,620° C, and an 18-gram nodule of yttrium metal was collected at 1,075° C on a skull of frozen electrolyte. Current efficiency was low, approximately 20 percent, because the metal reacted with the electrolyte at the elevated temperature.
Continuous Electrowinning of Cerium and Lanthanum
A 12-inch-diameter cell was designed and operated to demonstrate the feasibility of preparing cerium and lanthanum metals in a continuous operation. Both metals were collected on molybdenum and tungsten hearths and were tapped from the cell into ladles in an inert-atmosphere casting chamber.
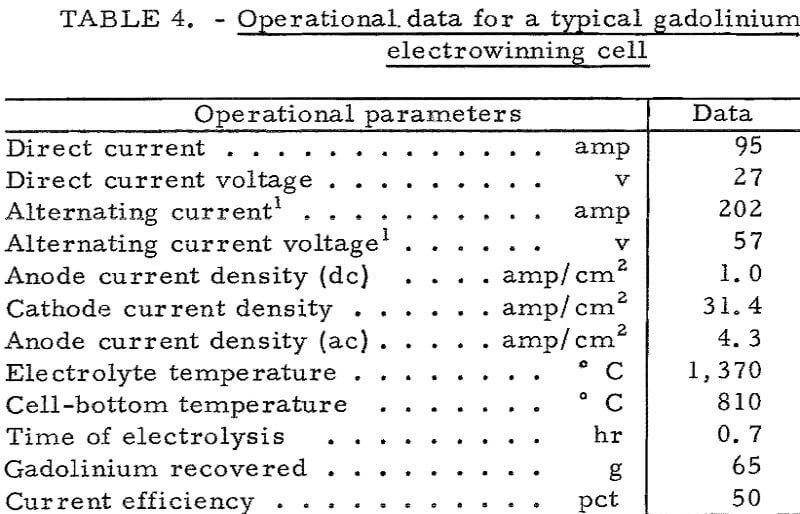
The basic cell design for electrowinning cerium is shown in figure 3. The graphite crucible had a 60° sloping bottom, completely lined with molybdenum sheet. A ½-inch-diameter molybdenum tapping pipe extended from the bottom of the molybdenum-lined crucible into the casting chamber, an integral part of the cell chamber. The adjustment of the cell-bottom temperature prior to tapping the metal was made with a separate heating unit located under the crucible. The single anode was a 22-inch graphite tube of 4-3/8-inch outside diameter and 3-inch inside diameter. The tubular anode also served as a feed tube for adding electrolyte or oxide. Molybdenum or tungsten rods of 0.4-inch diameter were used for the nine cathodes spaced evenly in a circle 1-1/8 inches from the anode surface.
In typical cerium experiments the liquid electrolyte consisted of 63 weight-percent CeF3, 16 weight-percent BaF2, and 21 weight-percent LiF. Electrolysis was carried out at a bath temperature of 850° C. All the necessary heat was provided by the electrolysis current of 785 amperes at 8.5 volts. Cerous oxide was fed to the cell at a rate of 30 grams per minute. After 4 hours, electrolysis was stopped, alternating current was applied to heat the
molybdenum tapping pipe by contacting the pipe with a molybdenum rod bus bar, and the liquid cerium metal was tapped into ladles. Electrolysis was repeated in this manner for 3 consecutive days. During this time, several tappings of metal were made.
The electrode arrangement used for electrowinning cerium was not satisfactory for lanthanum, because sufficient current could not be maintained to sustain the operating temperature in the cell. The difference between the ability to sustain the requisite bath temperature in cerium electrowinning, and the inability to do so in lanthanum electrowinning, may be explained by the different valence species that can exist in the two electrolytes. In the case of the cerium electrolyte both +4 and +3 valent cerium species were present. If the anolyte did not contain sufficient oxide to sustain the anode reactions, the cerous ion was oxidized to eerie ion, and the cell continued to operate at the same amperage. As the ceric ion accumulated in the electrolyte, the oxide became more soluble, and a balance between oxide availability and anode reaction was reached.
Lanthanum, however, has only the trivalent species. If the oxide is not available to the anode at a concentration sufficient to support the imposed anode reaction, the electrolysis rate slows and the temperature decreases. Intuitive reasoning resulted in reversing the electrode arrangement to have one central molybdenum cathode surrounded by eight graphite anodes. This design placed the anodes on the outside and allowed a greater proportionate volume of the electrolyte to serve as anolyte. Using this electrode arrangement, lanthanum metal was electrowon and tapped in a 4-day continuous operation.
Product Quality
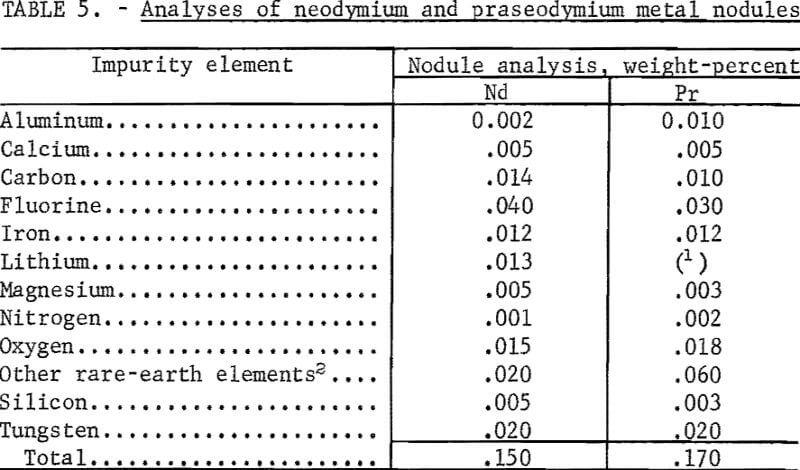
Examination of rare-earth metal nodules obtained from batch operation in the 6-inch-diameter cells showed small amounts of salt adhering to the nodule surfaces. The salt was removed and the nodules were sampled for analysis. The purity of typical nodules of neodymium and praseodymium is given in table 5. Most of the metallic impurities, except for tungsten from the cathode, originated in the electrolyte and feed materials. Microscopic examination of the nodules revealed small amounts of interspersed electrolyte, a source of the fluorine contamination. Analyses of didymium and ferroalloy nodules gave results comparable to those obtained for neodymium and praseodymium.
The carbon and oxygen impurity levels in gadolinium, dysprosium, and yttrium metals were considerably higher than those in neodymium and praseodymium. These high values were attributed to the reaction of the metals at elevated temperatures with carbon oxides produced at the anodes and oxide sludge in the bath. The design of the high-temperature cell, incorporating shallow immersion of fluted anodes and the maintenance of a thermal gradient between the zones of metal deposition and collection, helped to minimize contamination of the metals. For example, gadolinium metal won at 1,400° C and collected at 860° C contained 500 ppm carbon and 100 ppm oxygen, whereas metal won at the same temperature and collected at 1,000° C contained 10 times that amount of oxygen and carbon.
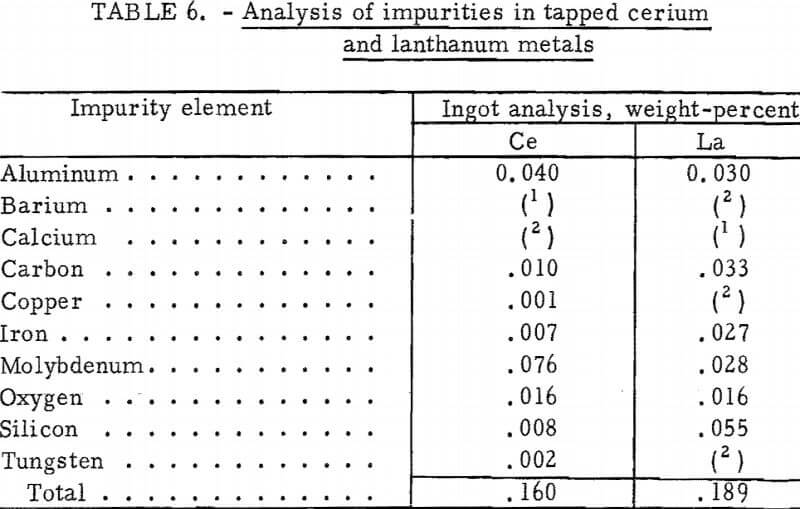
Analysis of the impurities in cerium and lanthanum metal ingots cast during continuous electrolysis experiments is given in table 6. Molybdenum, the major impurity in the cerium metal, came from the molybdenum hearth and electrodes. Lanthanum metal collected on a tungsten hearth contained <100 ppm tungsten.