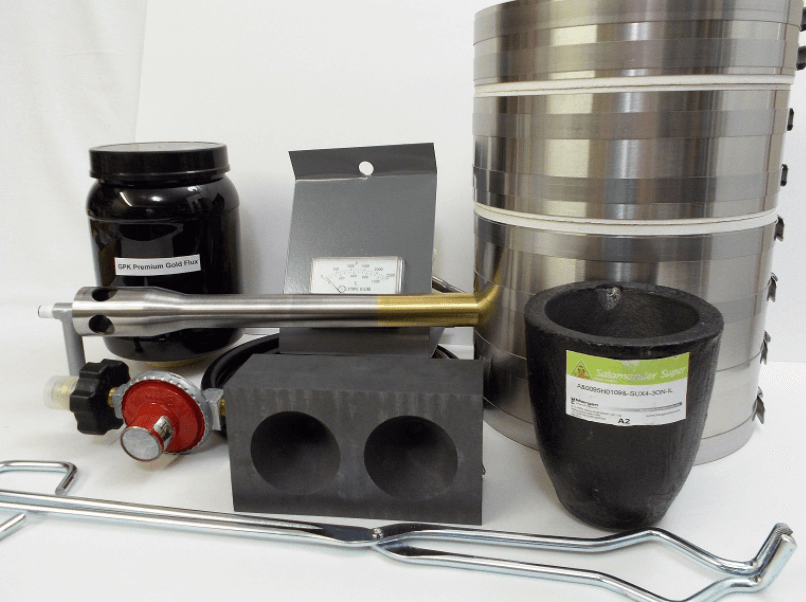
Showing all 11 results
Gold Melting Furnaces: A few years ago, the spotlight was after creating a microwave kiln for smelting metals and performing fire assays. The first product was a microwave kiln that was quickly followed with the creation of the 4″ which is called the Kiln.
The 4″ kit comes complete, minus propane and something to smelt, of course. The Kiln has seen fantastic success with more than 3,000 units sold over the last two years. That’s four Kilns a day and the demand is not slowing down.
Many have used the Kiln on many occasions and truly understand why it’s quite possibly the highest selling kiln of all time. It is fast, easy to use and in the long run can make a pour of any metal for pennies in cost. Practice makes perfect, and to do a handful of pour for a couple of dollars makes practice not only perfect but affordable as well. For the more experienced goldsmiths, one major drawback to the 4″ Kiln—its size. It is a small unit, highly portable and great for doing small pour.
We followed the massive success of the 4″ with the 8″, which is for all intents and purposes an industrial kiln. It handles a great deal more material than most of us in the prospecting realm will ever need, and it does take a specialized burner and a much bigger fuel source. For some, the 8″ is too big, that’s why the 6″ was born.
There are inherent dangers associated with almost any aspect of mining, and adding 2,000-plus degrees to the mix calls for great respect and attention to even the finest of details.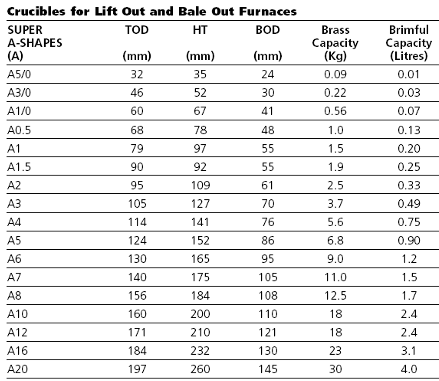
The first product was the microwave kiln, invented to perform simple and efficient fire assay work by the author of The Art of the Microwave Fire Assay.
The fluxes are designed for optimal performance, and the instructions list all the steps in creating excellent pours every time. You will find out rather quickly that what you thought were great recipes for flux didn’t really compare to what you will find here. Our flux just works better.
“Always treat everything like it is very, very hot, because it is!”
SAFETY:
- DO NOT place a furnace directly on a metal, wood or plastic table or workbench.
- Fire bricks or pavers are are great choice for covering a wooden workbench before setting down a furnace.
- Wear thick, leather welder’s gloves and in addition to safety glasses you could wear a flip down face shield as well.
- No loose clothing, and always smelt in a ventilated space!
- Stay Focused
There is nothing complicated about smelting metal with the 6″, which means you need to have unwavering presence of mind because complacency is dangerous.
Set the kiln base on a flame-retardant table and organized your work area up to use complete economy of motion. When using a kiln, you need to spend time in setting up the exact location of every step. Movement while pouring is calculated and precise.
Pros
- Easy to use for professionals and first-time users
- Extremely low cost to operate
- Fast, efficient smelting of all metals rated to 2200° F
- Choices in crucibles to use in the 6″
Since most of you will be using the 6″ in the most natural space, you best move to a garage and begin setting up to make a first pour. The 6″ setup is intuitive. But again, if you’re new to smelting, read the instructions a few times before lighting the fire.
Prepare your work environment. Have a place to set the chamber, a place to pour into the mold and a place to set the crucible are all that’s needed.
When you fire up the torches, place them into the orifices on either side of the 6″. You cannot just stick torches into the unit and let the fire go.
In appearance, the 6″ is the 4″, except larger, which is perfect for current Kiln users. Our kilns are designing to look and function the same.
Plus, those of you who are current 4″ users, the crucible designed for the 4″ fits perfectly into the 6″ for those smaller pours. Most importantly, though, is that those new to smelting will “get it” quickly.
The 6″ is hand built out of the same Alumina Silicate refractory sheet material used in all products and is neatly wrapped in stainless steel. As a kit, the 6″ comes to you almost complete. You’ll need to purchase a couple of small propane bottles that are readily available in any sporting goods, camping or plumbing supply store, and two Bernzomatic TS400 torches or other brands rated at 6,000 BTU each. These are easily bought in most plumbing departments, or online.
By design, all gas kilns have a slight angle to the entry port for the torch head. This allows the flame to create a cyclone in the bottom of the kiln that looks like the center of a tornado. The cyclone (or vortex) is an important step to achieve; it creates evenly distributed heat up the entire column of the kiln which smelts the metals in the crucible perfectly, leaving no hot or cold spots in the crucible.
The line of kilns are simple to operate and, with a little common sense, are safe. Anyone new to furnace work can do everything from setup to pour.
Stack the unit onto the base: Gloves on, place the center section and the lid on the base and let the unit start coming up to temp. Placing 31 grams of gold (that included black sand and some ground quartz) into a 4″ mini crucible and using a half a teaspoon of our Premium Gold Flux, you can set the crucible on the edge of the 6″ lid to warm up.
Again, gloves on, separated the unit, place the crucible into the 6″ and re-stacked the chamber section. When stacking the furnace, you have to ensure there is equal space around the crucible for proper heat distribution. Because of the wider chamber of the 6″, this is easily done and is safer than any other gas furnace. There is never a need to move the crucible after it’s in the flame. Simply adjust the center section of the chamber to ensure proper space, and set the lid.
Place the pour mold onto the lid of the 6″ to heat up and wait for the smelting. In less than eight minutes, you have a look into the crucible and carefully look into the chamber through the vent hole to see everything in a clear molten state.
For larger pour using the standard 6″ A05 crucible and say two troy pounds of gold with flux, you will still be pouring gold in under a half-hour from the time of lighting the torches.
Pouring Gold
Separate and lift the chamber straight up and over, and sit it on the predetermined spot. Then, firmly grasped the crucible with the kit’s tongs, lift and move it to the mold, pour purposely and sit the crucible down in another predetermined spot. Then, re-stack the kiln, turned off the torches, and then wait for the pour to cool.
What drops out of the mold should be a perfect six-sided coin weighing in just a few grains less than what you started with.
A electronic test of the coin may show it to be 99.99 fine gold. The impurities, as expected, will be trapped in the flux or destroyed all together in the smelting process.
By smelting and pouring your own gold, its purity can be checked instantly with either an acid scratch test or by electronic testing equipment that has become affordable to just about anyone these days. With a gold bar in hand, you’ll know exactly what you have and the value you can expect to receive.
The alumina silica refractory material of our gold processing kilns is good for up to 2300 degrees Fahrenheit. Once the material reaches the targeted melting temperatures, the refractory material will shrink approximately 5%, which will create small cracks in the material when it cools. However, cracking is not detrimental to the operation of the kiln or the life expectancy of the kiln.