Table of Contents
How to Remove Copper from Fine Gold
Prevention of copper entering the final bullion has been the principal objective of all experimental work performed on zinc slimes in the goldroom. Above all other metals encountered during treatment, it is the most difficult to separate from gold and silver.
Broadly, two methods are possible for elimination of copper. The first is, to prevent its entry into the Merrill-Crowe precipitation plant in the cyanide solutions. Practically no experimental work has yet been done towards this end, although the possibility has not been dismissed. The second method is to remove the copper after it has been precipitated on zinc dust with the gold and silver.
Current practice, as already described in Parts I and II, involves converting most of the copper present in the slimes into the form of a lead-copper matte, which requires a considerable amount of further treatment to recover the gold and silver it contains. Moreover, the bullion obtained from the initial smelt still carries approximately-10% of copper, so that the process cannot be considered ideal.
Aciding of raw zinc slime with dilute sulphuric acid was the earliest treatment tried to overcome the copper removal problem when it first appeared. It was abandoned because of non-success. Granulation and sulphuring of bullion were then adopted from sheer necessity, being followed, as a natural development, by the pyrite matte forming smelt of current practice.
Recent experimental work has been re-directed towards acid treatment of both raw and roasted-slimes. Intensive testing has indicated that when suitable equipment, that will withstand the action of the acids used, can be obtained, the method will be applicable on a plant scale. Two advantages should accrue from its use: A higher grade of final bullion, and no matte formation with its attendant complications.
Some form of acid treatment, then, appears to be the trend for the future, but two other methods have been partially explored.
When copper first became troublesome in the plant, small smelts were run using manganese dioxide in an attempt to oxidise the copper and slag it off. Three of the crucibles used contained graphite in their composition, one was almost free from graphite. The grade of bullion obtained from these trial smelts indicated in no uncertain manner, that to smelt in a furnace lined with a reducing agent would vitiate any attempts to slag off the copper. Efforts have therefore been made over a number of years to obtain a furnace liner containing no reducing agent, that will withstand the action of fluxes and oxides, but unfortunately so far none has been found suitable.
Fusion with salt cake is the other method which has been tested as an alternative to the matte process. Fusion is slow, however; extraction of the soluble sulphates is not as simple as it first appears, and although the bullion grade obtainable is high, the method does not appear to possess enough advantages to merit further work or application.
- Past Gold Refinery Practice
- Roasting
- Smelting
- Barring Furnace
- Granulation & Sulphuring of Bullion
- Best Gold Refining Methods
- Silver & Gold Smelting Practice
- Amalgamation
- Flotation Strakes
- Roasted Concentrate Strakes
- Retorting of Amalgam
- Recovery of Metals from Mattes and Slags
- By Products of Gold Refinery
- Slags
- Mattes
- Lead Bath Treatment of Matte
- Reduction of Mattes to Metals
- Cupellation of Base Bullion
- Treatment of old Furnace Liners
- Recovery from Cupellation Oxides
Acid Treatment of Fine Gold
Laboratory scale tests were commenced to try to find an acid or combination of acids with which to treat the zinc slimes, so that on smelting with a normal silica-borax flux, they would yield an acceptable grade of bullion without matte formation. From a practical point of view, whether the slime was to be roasted or not, before or after acid treatment, was not considered a major issue, although it was recognised that elimination of roasting would simplify process detail by reducing the number of stages.
Preliminary Tests
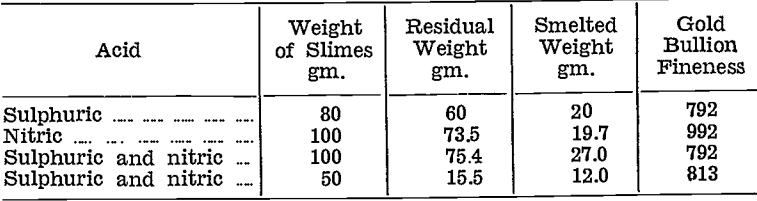
Preliminary trials were made on raw and roasted zinc slimes from the main Chaffers plant, and on raw slimes from the Chaffers retreatment plant, using nitric and sulphuric acids, alone and in combination.
The procedure in most cases was to add water first, then to make successive small additions of the particular acid to initiate and maintain reaction. When action was complete, the residue was filtered, washed, dried and smelted. The table below summarises results from this series of tests.
Conclusions drawn from the tests were:—
- Treatment of the Chaffers plant roasted slimes, with nitric acid alone, showed promise.
- Treatment of the Chaffers retreatment plant raw slimes with either sulphuric or mixed sulphuric- nitric acids, was a decided advantage.
Confirmatory tests were made on further samples of Chaffers plant slime. Some of the plant raw slime was taken and roasted in the laboratory electric muffle furnace to ensure good roasting.
Roasting Test 1:
Sample weight…………………………………………………………….150 gram.
Roasted in the muffle furnace at 650°C. for 2½ hours with regular rabbling.
Calcined weight……………………………………………………………100 gram.
Percentage of original weight………………………………………..66.7 gram.
Roasting Test 2:
Sample weight…………………………………………………………….150 gram.
Added nitre 20 % by weight…………………………………………..30 gram.
Roasted as for Test 1
Calcined weight…………………………………………………………..161 gram.
Percentage of original weight………………………………………89.5 gram.
Plant Roast:
For the same day, 19/6/1944
Percentage of original weight…………………………71.0 gram
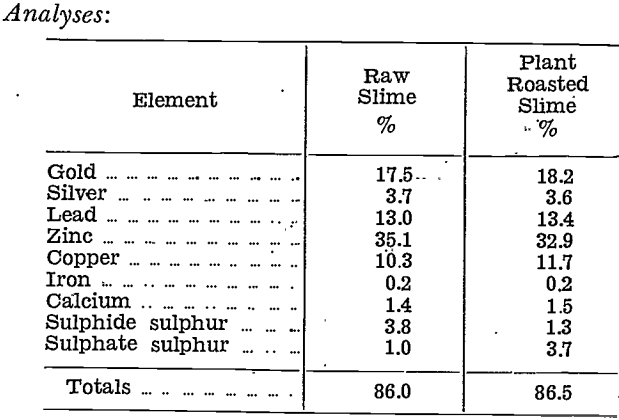
Base metals, calculated to oxides in the plant roasted slime, totalled 98.4%.
Treatment of Roasted Slime with Sulphuric & Nitric Acids
Aciding tests were then made on the laboratory roasted, and plant roasted slimes. Solutions from the acid treatment were analysed to determine the effectiveness of copper removal, and the slags resulting from smelting were, assayed for copper, because for plant practice they should be low in copper if they are to be cyanided. Table 9 summarises the tests.
Again, nitric acid appeared to give the best results, but it should be noted that smelting was done in clay crucibles, and the weight after aciding had not been reduced sufficiently to be able to use a clay lined crucible on a plant scale.
A larger scale test was next made with slime roasted under plant conditions. Details are as follows:—
Test (a): Sample taken 6/7/1944:
Plant results: 3.1 oz. of bullion per lb. of slime, bullion assayed 815 fine gold, 62 silver, 123 base metal.
Sample weight………………………………………………1,816 gram.
Added manganese dioxide……………………………….363 gram.
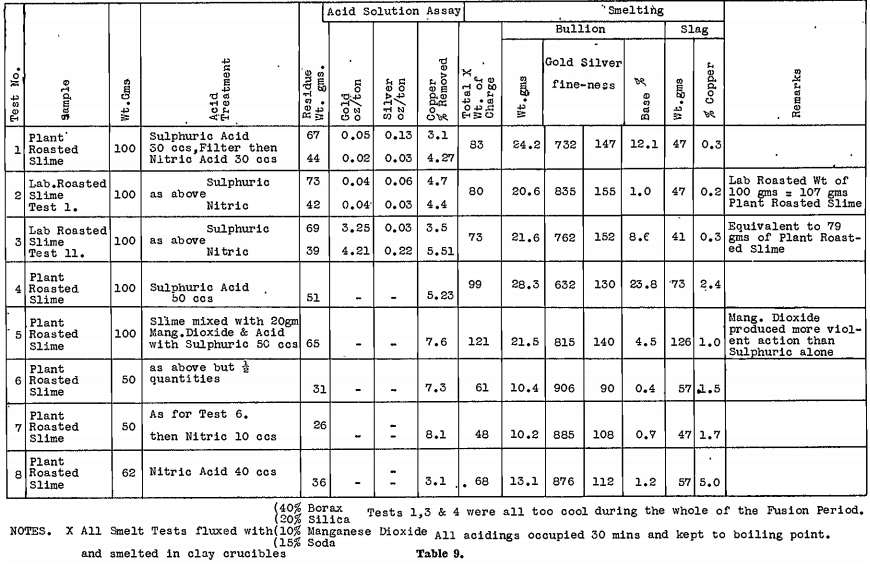

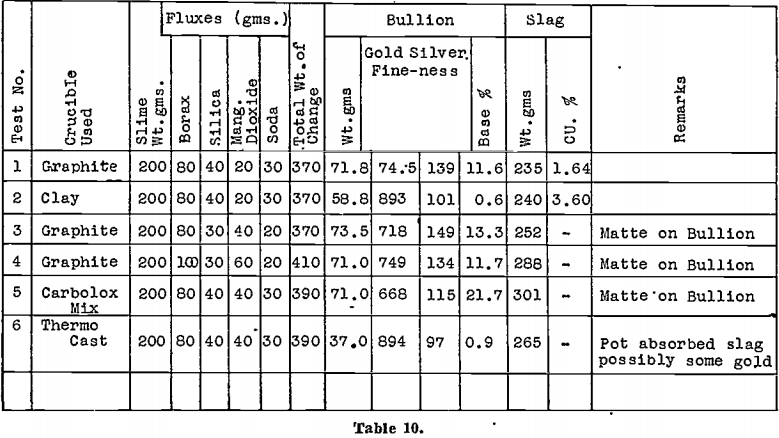
The residues from both tests were divided, and smelting tests made, using several different lining materials for the crucibles. Results are shown in Table 10.
The tests clearly indicate that these acid treatments are not sufficient without the use of clay lined smelting crucibles.
Treatment of Raw Slime with Hydrochloric & Sulphuric Acids
Exploratory tests on raw zinc slimes, using hydrochloric and sulphuric acids, with and without manganese dioxide, were made. Details are as follows:—
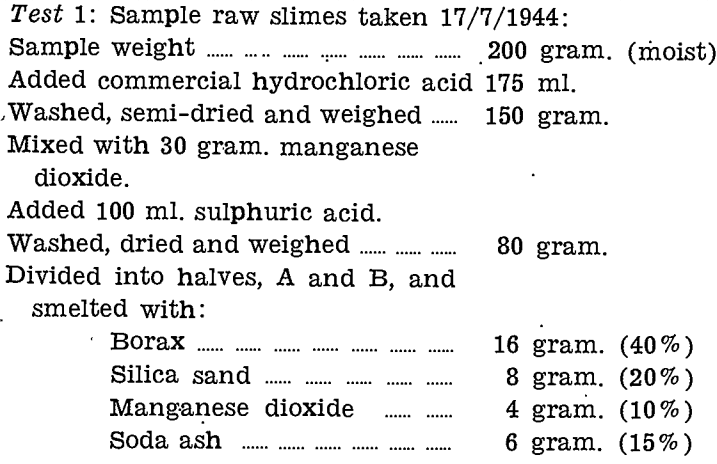

Test 3: Sample weight………………………………………….10 gram.
Acided with 4 gram, manganese dioxide and 10 ml. hydrochloric acid, dried and weighed………7.7 gram.
Acided with 6 ml. sulphuric acid, dried and weighed……………………..4.2 gram.
Wrapped in own weight of lead and cupelled. Left impure mass. Added more, lead and cupelled to a good bullion button, weighing…………………………………..1.475 gram
Test 4: Sample weight……………………………………………………10 gram.
Acided with 10 ml. hydrochloric acid, dried and weighed………………3.8 gram.
Acided with 6 ml. sulphuric acid, dried and weighed……………………3.5 gram.
Wrapped in own weight of lead and cupelled. Gave a button of poor-looking bullion with black copper oxide coating, weighing…………………………1.995 gram.
Tests 5 and 6: Sample taken 3/8/1944:
Preliminary test done first to check Test 4, using 3 gram, hydrochloric acid, followed by 2.6 gram. sulphuric acid: residual weight……………………………2.6 gram
Test 5: Sample weight………………………………….250 gram.
Boiled 30 minutes with 250 ml. commercial hydrochloric acid, decanted and washed by decantation with hot water, added 20 gram, manganese dioxide and 125 ml. commercial sulphuric acid. Boiled 30 minutes, filtered, washed, dried and weighed………………………77 gram.
Weight of lead chloride recovered from solution……………………22 gram.
Percentage of original weight………………………………..38.5 gram.
Test 6: Sample weight………………………….248 gram.
Procedure as for Test 5, but no manganese dioxide used. No blue colour was obtained in the sulphuric acid treatment:
residual weight…………………………………….75 gram.
Percentage of original weight………………30.2 gram.
Residue from Tests 5 and 6 were combined and divided into two halves, (A) and (B), treated as follows:
Sample (A): Sample weight…………………………….50 gram.
Smelted with:
Borax glass………………………………..25 gram.
Silica………………………………………….5 gram.
Manganese dioxide……………………10 gram.
Soda ash……………………………………5 gram.
Fluor spar………………………………….5 gram.
Bullion weight………………………..33.3 gram.
Slag weight……………………………….66 gram.
Bullion Assay:
Gold………………………………………..619
Silver………………………………………137
Base metal………………………………244
Comments: Good fusion, and fluid slag.
Clay crucible used.
Sample (B): Sample weight………………………………105 gram.
Roasted in laboratory furnace at 700° C. with frequent rabbling: Residual roasted weight……….77 gram.
Smelted with:
Borax glass……………………………….31 gram.
Silica……………………………………….10 gram.
Manganese dioxide…………………..16 gram.
Soda ash…………………………………..8 gram.
Bullion weight………………………..39 gram.
Bullion Assay:
Gold……………………..857
Silver…………………….112
Base metal……………..31
Comments: Good fluid slag.
Clay crucible used.
Summarising this series of tests, treatment of raw zinc slimes with hydrochloric acid alone, or followed by treatment with sulphuric acid and manganese dioxide,, was no more effective in removing base metal than treatment of roasted slime with mixed nitric and sulphuric acids.
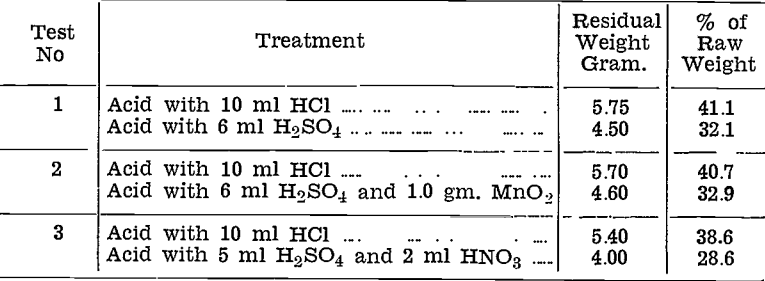
Treatment of Roasted Slime with Hydrochloric Acid
followed by Mixed Nitric-Sulphuric Acids.
Small scale tests were made on roasted slime, in two stages. Commercial hydrochloric acid was used first, then after filtering and washing, the residue was further treated with mixed diluted nitric and sulphuric acids.
The table below shows the results. The weight of sample taken in each case was 10 gram., equivalent to 14 gram, of raw slime.
Test No. 3 was repeated on a larger scale with a sample of plant roasted slime taken on 17/7/1944.
Repeat Test 3: Weight of sample 302 gram.
Added 302 ml. commercial hydrochloric acid; filtered, washed and weighed…………………..183 gram.
Acided with 151 ml. sulphuric acid and 60 ml. nitric acid; filtered, washed and weighed………..120 gram.
Percentage of roasted weight……………………..39.8 gram.
Percentage of raw weight……………………………28.4 gram.
Combination: Acid – Roast – Acid
Aciding of raw slime with hydrochloric acid reduced the weight of slime to 38.5% of the original weight of raw slime taken, (see Test No. 5), by removing zinc and lead as chlorides. It was decided to attempt the further removal of base metals by acid treating slimes, roasted after the hydrochloric acid treatment.
Accordingly the following test was carried out:—
Sample taken 14/8/1944
Slime smelted in the plant on the same day yielded 2.0 oz. of bullion per lb. of raw slime.
The bullion assayed:
Gold……………………………………………800
Silver…………………………………………..60
Base metal…………………………………..140
Sample weight…………………………495 gram.
Acided with 495 ml. hydrochloric acid, semi-dried weight, 300 gram.
Rewashed with boiling water, filtered, dried and weighed…………………120 gram. (24.3%)
Roasted in the laboratory muffle furnace for 1 hour at 700°C. weight after roasting……..108 gram. (21.8%)
Acided with 100 ml. sulphuric acid and 20 ml. nitric acid. Washed free from copper, filtered, dried
and weighed…………………………………..74 gram. (14.9%)
Assayed Residue:
Gold………………………………………..770
Silver………………………………………108
Base metal……………………………….122
Smelted the residue in three portions, A, B, and C, as below.
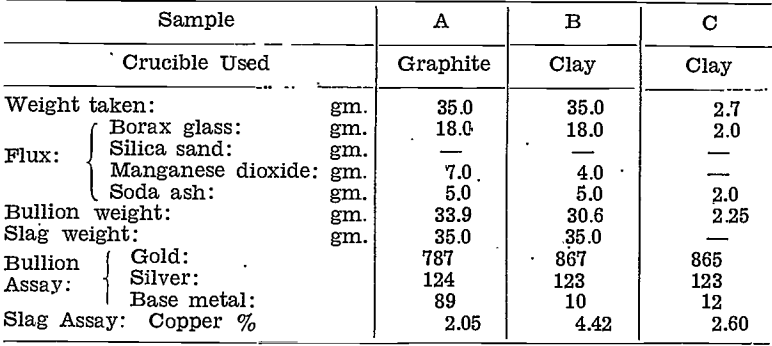
A repeat test was made to confirm the above results, the procedure being the same, except that no nitric acid was used.
Repeat Test: Sample taken 23/8/1944:
Sample weight……………………………………860 gram.
Acided with 860 ml. commercial hydrochloric acid, filtered, washed, dried and weighed………..216 gram.
Roasted 1 hour at 700°C. and weighed……………………………216 gram. (25.1%)
Acided with 200 ml. sulphuric acid, filtered, washed, dried and weighed……………………110 gram. (12.8%)
A small sample of residue from the acid-roast-acid treatment was assayed, and the balance smelted:—
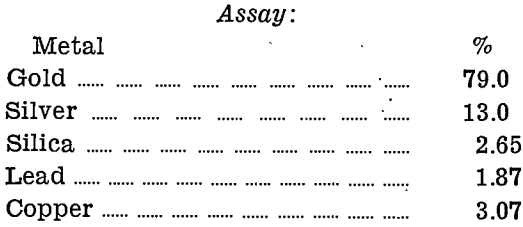
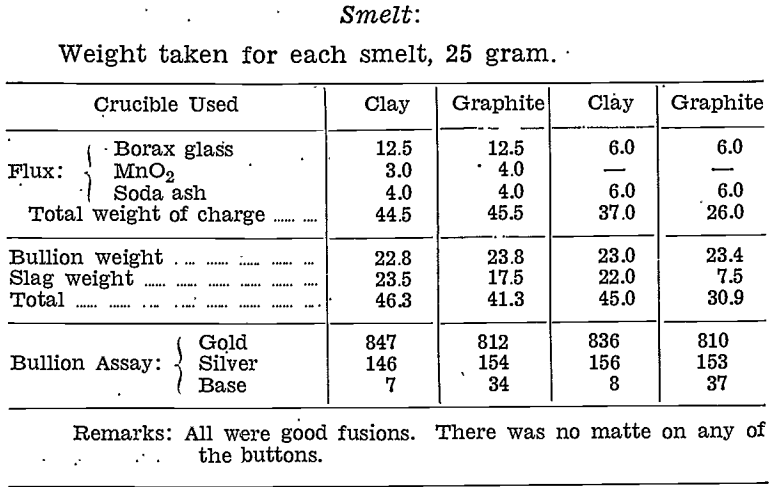
Conclusions drawn from the whole series of tests, using any or all of the three mineral acids, sulphuric, nitric, and hydrochloric acid, are as follows:
- Aciding of raw slime with hydrochloric acid followed by sulphuric acid, and then roasting reduced the weight of slime to 30.8 % of its original weight.
- Roasted slime treated with hydrochloric acid, followed by sulphuric-nitric acid mixture, reduced the weight to 28% of the original weight of slime.
- Raw slime when treated with hydrochloric acid, then roasted and treated again with sulphuric acid, was reduced in weight to 12.8% of the original weight.
- Only the acid-roast-acid method gave a residual material which could be smelted in a silicon-carbide lined furnace and yet yield a bullion of sufficiently high grade to warrant the extra treatment.
- Advantages to be gained by this last acid treatment are:
(a) The weight of slime remaining to be smelted is small compared with the original weight.
(b) Cost of fluxes would be low for this small weight.
(c) Bullion grade is high, even when using a carbon containing furnace.
(d) There is a better chance of finding a non-carbon lining that might resist slagging, since the treatment removes most of the zinc, the metal which hitherto has rendered high- alumina linings unsuitable.
(e) Lead chloride is easily recoverable from the hydrochloric acid solution, and could be used to replace purchased lead nitrate at present fed to the Merrill precipitator. As lead chloride has a steep solubility-temperature curve, the solution would need to be kept warmed to a minimum of 40°C., but this is neither difficult nor expensive.
(f) Copper is removed by the sulphuric acid wash as copper sulphate, which could be used in the flotation section of the plant. - Disadvantages and difficulties of the acid-roast-acid method are:
(a) Removal of copper is only achieved after the other base metals have been dissolved out.
(b) The precipitate is subjected to three treatments before smelting. As the gold content increases progressively, so do any losses.
(c) Materials of construction to resist the action of hot hydrochloric acid are not readily obtainable.
(d) Washing out of lead chloride must be well done with an adequate supply of boiling water.
Treatment with Ferric Sulphate
Preliminary laboratory scale tests have been made of the method described in a paper by Hedley and Kress, whereby copper is dissolved directly from raw slime by agitation with a ferric sulphate solution acidified with sulphuric acid. The authors also found that preliminary leaching with sulphuric acid effected economy in the use of ferric sulphate by dissolving first easily soluble metals like zinc.
This method appears to have much in its favour compared with any of the other treatments so far discussed, for several reasons:—
- Copper can be dissolved without first removing other base metals, as in the acid-roast-acid method.
- The solutions used are not highly corrosive like hydrochloric acid.
- Roasting is not necessary from the point of view of copper removal, although it may prove advantageous for the subsequent smelting operation.
- Copper metal can be readily recovered, together with any gold which dissolves during treatment of the slimes with ferric sulphate.
- Although lead would not be recovered, the intrinsic value of the lead, is small, and the process does not call for an expensive acid to dissolve it.
The procedures adopted in this experimental work followed much the same course as in the original work by Hedley and Kress, viz.: Small samples of zinc slimes were treated at room temperature with the solvents being studied, for comparative periods of time, in open bottles agitated on rollers. The samples were then filtered, and washed, the residues were weighed, and the solutions analysed for copper.
Series 1. — Three tests were made, using 5 gram, (dry weight) of moist, lumpy slimes, which contained 10% of copper. Each sample was mixed with 50 ml. water and 2 ml. of sulphuric acid (sp. gr. 1.8), the required amount of ferric sulphate was added, and the bottles containing the mixture agitated for 1½ hours. The amount of ferric sulphate was the only variable in this set of tests, 15, 10, and 5 gram, being used.
The lumps of slime failed to break up during agitation, so that extraction was not as high as it might have been.
Results are tabulated below with those from the succeeding series.
Series 2. — In this series, three 5 gram, samples of fine dry slime were treated similarly to those of Series 1, except that the agitation time was increased to two hours. Extraction was higher and more consistent.
Series 3. — In the last series, each of three 5 gram, samples, similar to those of Series 2, were first agitated for ½ hour with 4 gram, of sulphuric acid and 50 ml. of water before adding ferric sulphate, 8, 6, and 4 gram., respectively, then-agitating for 1½ hours.
Finally, 32.49 gram, of slime were agitated with 320 ml. of water and 24.4 gram, of sulphuric acid for ½ hour, then 32.5 gram, of ferric sulphate were added and the mixture agitated for a further 1½ hours.
Extractions were lower than in Series 2. Comparative results are tabulated below:—
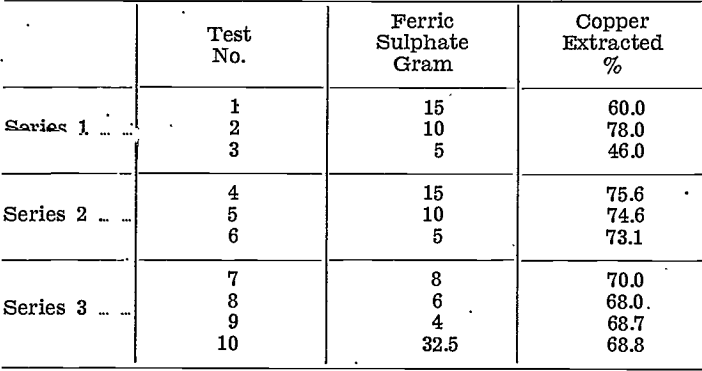
Copper extraction compares with the results of Hedley and Kress, for their low-copper (4.5 %) slimes.
Further experimental work is necessary before deciding whether the method can be successfully applied on a plant scale.
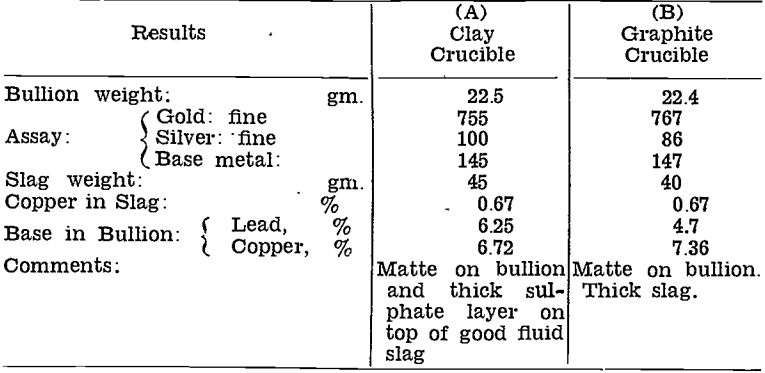
Smelting in Furnaces Lined with Carbon-Free Refractories
When copper first became troublesome about 1936, small scale tests were made in the gold-room on the effect of adding manganese dioxide to the flux, in an effort to oxidise the copper and slag it off. Four charges were run, three in Salamander crucibles, one in a Salamander crucible with a clay liner. All the graphite-containing crucibles yielded low grade bullion, whereas the clay-lined crucible gave a very high grade bullion button. The conclusion was drawn that carbon from the crucible caused reduction of copper from the slag into the bullion, and that manganese dioxide was incapable of preventing this from happening.
Efforts have been made ever since to obtain a commercial product containing no carbon or reducing agent, suitable for lining the plant furnaces, but so far without success.
The first samples tested were obtained from Nonporite Ltd., and Newbold Refractories Ltd., late in 1939. Enough of each was procured to line a small Salamander crucible.
The ground, moistened refractory was rammed into position by hand to form a layer about 1 in. thick around the sides and bottom of the crucible, being then allowed to dry out thoroughly before use by stacking above the brickwork of the muffle furnace in the gold-room.
Crucibles so prepared were annealed carefully by heating slowly in the well furnace to a red heat.
One sample of roasted slime was divided into six equal parts, and smelted with the same flux proportions for each. Results are tabulated below:—
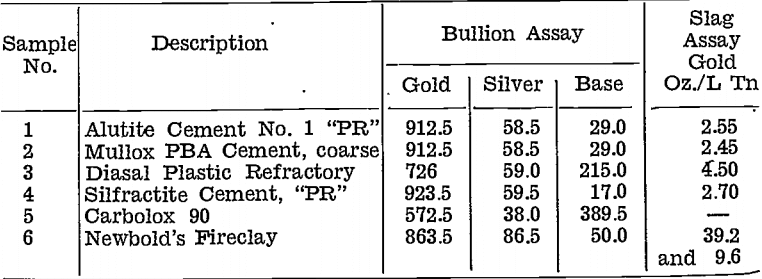
The liner corroded very badly with Sample No. 3, and the charge came in contact with the graphite crucible, hence the bullion grade was much lower than with the other samples.
Sample No. 5, Carbolox 90, was the original proprietary silicon-carbide supplied with the Wabi furnaces. Decomposition of the carbide caused copper to be reduced and to enter the bullion.
Slags from the specially lined crucibles were all low in value, although not as fluid as they would have been if no corrosion had taken place.
No tests were made on any of these materials on a plant scale. Confirmation of the reducing effect of carbon-containing furnace lining, and on the other hand, of the advantages that would result from using a carbon-free lining have been obtained on several-occasions since these early tests. See Table 10.
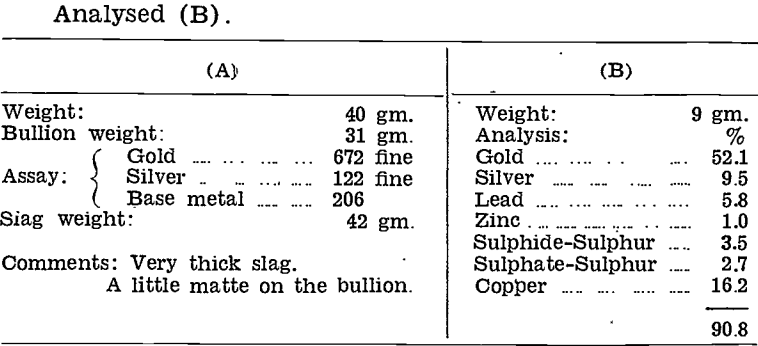
Tests on a plant scale were made in December, 1942, using a high-duty andalusite lining supplied by H. L. Brisbane and Wunderlich Ltd., Perth, W.A. The first, liner was made up in special shapes to fit the Wabi furnaces from a plaster cast pattern, and cemented into position with refractory cement.
Three charges in all were run in this furnace before the lining failed by corroding away at the slag line. Results were as follows:—
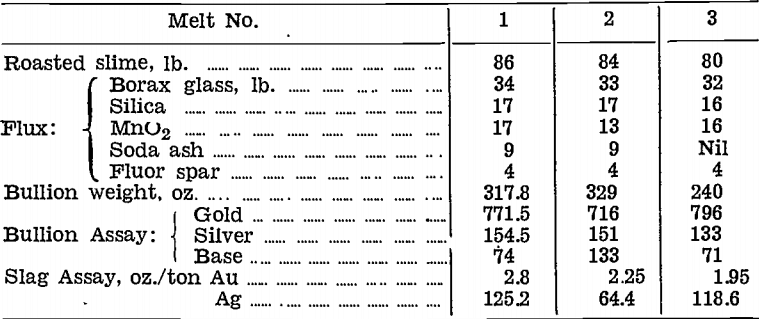
Actual smelting time for each charge was 1¼ hours. The whole life of the liner, including preheating time, was 7 hours 10 minutes.
Examination of the lining before Charge No. 3, revealed a large crack across the bottom and a small crack of no consequence at the pouring lip. The lining was cut badly at the slag line to a depth of 1¼ to 1½ in., and fairly evenly over the rest of the working surface to a depth of 1 in. There were no signs of deterioration above the slag line.
During smelting of Charge No. 3, a Brisbane and Wunderlich “Bristile” high-duty andalusite brick, and a New- bold’s “Kyanite” brick were thrown into the smelt. They were recovered just before pouring and examined. The Bristile brick was badly pitted and slag had penetrated some distance into it. The kyanite brick was slightly corroded on the surface, but there was no slag penetration.
A second high-duty andalusite lining was tested, with similar results to the first. Both linings were excellent in appearance after one charge had been run, but excessive corrosion started during the second run, indicating that there was an outer layer of skin of good resistance, covering a porous interior which was readily slagged.
Following the failure of these liners, it was decided to suspend further test work, temporarily at least, and proceed with investigations on the recovery of gold and silver from the mattes produced by the semi-pyritic smelting process in use at that time.
Fusion of Gold Slime with Salt Cake
Six laboratory tests were made in October, 1944, on the fusion of gold slimes with sodium hydrogen sulphate, the salt-cake obtained as a by-product in the manufacture of nitric acid from saltpetre.
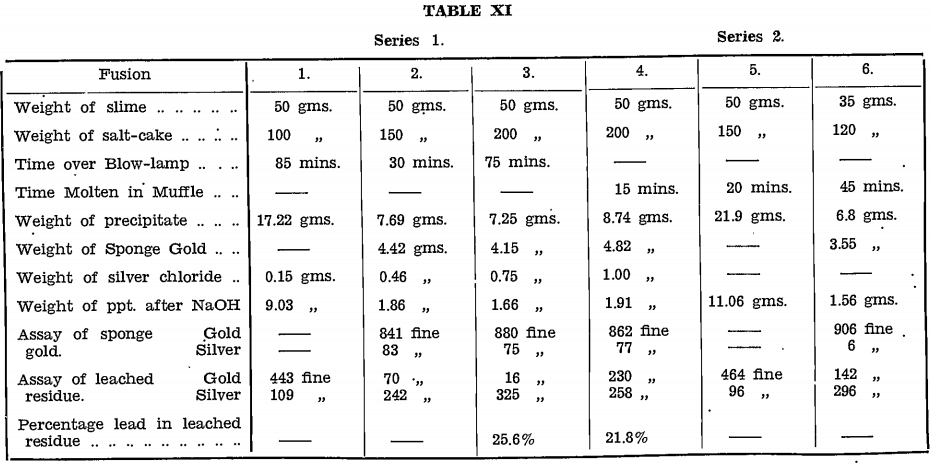
In Series 1, three 50 gram, samples of moist raw slime were mixed with salt-cake in the proportions:—
Fusion 1.—2 parts salt cake to 1 part zinc slime.
Fusion 2.—3 parts salt cake to 1 part zinc slime.
Fusion 3.—4 parts salt cake to 1 part zinc slime
and heated over a kerosene burner, “Primus” type. As there was no sign of action after ½ hour, the samples were melted over a kerosene blow-lamp. The charge in each case swelled while pasty but was quiet when fused.
Fusion 1 fused with difficulty to an earthy brown mass.
Fusions 2 and 3 were blue during the pasty stage, changing to dark green when molten, and blue again on cooling. Small lumps of spongy gold could be seen throughout the mass.
Each melt, after cooling, was dissolved in about 700 ml. of water, boiled for ½ hour, then decanted through filters. With Fusions 2 and 3, the light flocculent precipitate of silver chloride was decanted away from the sponge gold through separate filters, but with Fusion 1, no such separation could be effected.
The precipitates were leached by boiling with twice their weight of sodium hydroxide.
Results are given in Table II, and with those of Series 2, the procedure for which follows:
In Series 2, the samples were melted under controlled temperature conditions in the laboratory electric muffle furnace.
Fusion 4 was run to determine the temperature required for fusion. The charge was placed in a cold furnace and the
temperature gradually raised until the charge melted at 600°C. The mass was similar in appearance to Fusions 2 and 3. Subsequent treatment was as for Series 1. Results were slightly higher, because the slime had dried out somewhat over a 24 hour interval, so that the original 50 gram, actually contained less water and more metal than Series 1 samples.
Fusion 5 was placed in the muffle which had been previously heated to 400°C., and raised quickly to 625°C. It was held at that temperature for 20 minutes, then poured. Part was granulated — not very successfully, as it exploded — by pouring into water. The fused mass was an earthy brown colour and was difficult to dissolve. The sponge gold could not be separated from the flocculent precipitate by decantation.
Fusion 6 was placed in the muffle at 400°C., brought quickly to 625 C., held at that temperature for 45 minutes, then poured. The fused mass, when cold, was a dark olive-green colour. It dissolved easily and a good separation was made by decantation.
A large-scale test, 50 lb. of slime, was next made on 23rd November, 1944, using an oil-fired, cast iron melting pot, and an improvised, rather poor leaching apparatus.
Plant results for the same day were: 764 lb. slime yielded 879 fine oz. of gold and 169 fine oz. silver, including values contained in the matte and slag. Hence 50 lb. taken for. the experiment should have contained approximately 57.5 fine oz. gold, 11.1 fine oz. silver.
The 50 lb. sample of slime was mixed with 170 lb. of salt-cake, and fed into the previously heated melting pot at 10.40 a.m. Violent action took place with some spitting. The burner was turned off until 11.05 a.m.
Melting then proceeded unevenly, as one side only of the pot could be kept hot, until 1.45 p.m., when all action had ceased, and the burner was turned off.
The fused mass was ladled out, cooled, and placed in a tub for dissolving. Water was added and the resulting liquor withdrawn from time to time, being transferred to a drum for settlement. Fresh water was added after each withdrawal of solution, a total of about 40 gal. being used.
Next morning, the liquors were decanted and the undissolved fine sludge recovered.
Two products were obtained for further treatment, gold sponge, and insoluble lead sulphate.
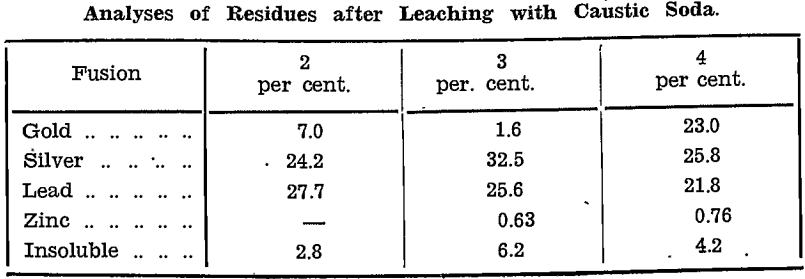
Gold Sponge was boiled with 2 lb. caustic soda to dissolve lead sulphate. Some silver chloride was present, due to sodium chloride in the tap water used.
The extracted gold sponge, after filtering and drying, weighed 69 oz. It was melted with a little borax in a graphite crucible, yielding 56.5 oz. of bullion assaying:—
Gold………………………………………….989, equivalent to 55.88 fine oz.
Silver……………………………………………9, equivalent to 0.51 fine oz.
Base metal……………………………………………………..2
Insoluble Lead Sulphate: The clear liquor was decanted and the solids filtered. The solution contained neither silver nor copper. Owing to an iron drum being used, the solids contained cement copper. The 14½ lb. obtained assayed:—
Gold……………………………………268 oz. per ton, equivalent to 1.7 fine oz.
Silver…………………………………1,212 oz. per ton, equivalent to 7.8 fine oz.
Lead…………………………………..22.25%, equivalent to 32.6% PbSO4
Copper………………………………….15.0%
On smelting in a clay crucible, this slime yielded 50.5 gram, of bullion assaying:—
Gold……………………………………858, equivalent to 1.39 fine oz.
Silver………………………………….136, equivalent to 0.22 fine oz.
Base metal……………………………………6
Total gold recovered………………….57.27 fine oz.
Total silver recovered………………….0.73 fine oz.
A second test, using 20 lb. of slime, was made on 4th December, 1944, after alterations had been made to the furnace brickwork in an effort to make heating of the cast iron melting pot more even.
Plant returns for the same day were: 1,136 lb. of slime yielded 1,228 fine oz. gold and 198 fine oz. silver, including values present in the matte and slag. Hence 20 lb. taken for the experiment should have contained 21.6 fine oz. gold and 3.7 fine oz. silver.
On this occasion the sample was not mixed with salt-cake, but added to the melting pot in layers. Some spitting still took place, however. The weight of salt-cake used was 60 lb. Time of melting was 3 hours 5 minutes.
Treatment was similar to the previous test, except that precipitation of copper was prevented by using an earthenware vessel instead of a steel drum for the decanted solution. Results were as follows:—
Gold sponge:
Final weight after leaching……………………………29 oz.
Yielded bullion…………………………………………….25 oz.
Bullion assay:
Gold……………………………………………924, equivalent to 23.1 fine oz.
silver…………………………………………..15, equivalent to 0.35 fine oz.
Base metal………………………………………………..62
Solution: No silver in solution, therefore discarded.
Lead Sulphate Residue: Weighed 6½ lb. This was thrown into the plant slag.
Conclusions drawn from the whole set of tests are:—
- Fusion with salt-cake removes nearly all the base metals, leaving gold sponge, which can be melted and poured directly into bars of high grade bullion, and a lead sulphate precipitated, which can be readily smelted in a clay-lined crucible to yield high grade bullion.
- The melt can be performed in an iron furnace that requires no high-cost refractory liner.
- Melting is slow.
- The molten sulphates are difficult to handle as they cannot be granulated.
Conclusion
While it is realised that the formation of a matte and the subsequent retreatment of the products from such a procedure is not ideal, it is still current practice in the gold-room.
It can be considered as a compromise method of treatment until the ideal method is found.
The latter part of this paper has dealt with attempts to find this ideal method, and work is still continuing with that object in view.
The method desired should fulfil the following requirements:
- All bullion produced should be higher in grade than the minimum of 98% total gold plus silver, set by Government regulations, to avoid Mint refining charges.
- No by-product to be produced containing quantities of payable precious metals that cannot be treated on the mine.
- The whole procedure to be quick in the return of bullion, to avoid locking up values in the smelting room.
- It must be safe from the operatives point of view, and not unduly arduous or exhausting.
- It must be sound economically, so that the cost for smelting and refining does not exceed the benefits to be derived from lessened Mint charges.
The matte smelting procedure in use at present, and described fully in this report, when analysed on the above lines is found to fulfil the conditions set out in (2), (3) and (4), in that no by-product has to be shipped for recovery of values, that it is a quick and easy smelting procedure with no difficulty or arduous operation, and that it does not require highly skilled operatives to watch continuously any step in the whole procedure.
