Table of Contents
Recovery of Metals from Mattes and Slags
Long ago, mattes and slags, which were the end products of the matte smelting process described in Part I, were sent to Port Kembla for final recovery of their metal content. Approximately 15 tons of slag and eight tons of matte used to be shipped yearly. Shortage of shipping and rising charges for treatment of these products since the war began, caused the decision to be made to attempt their further treatment in the gold-room.
Slags were first considered, and an alternative to the normal method involving the use of mercury, which was in short supply, was sought. Although no alternative to amalgamation was found, ordinary cyanidation after amalgamation obviated the need for sending them to the smelter.
Matte treatment proved to be a much more complex and difficult problem. Much experimentation was carried out on what has been termed the “Lead-bath”’ method, in which lead is added to the matte. The two materials are melted together, the lead forming a layer below, and the matte is left in contact with the lead bath for some time in order to collect the precious metals. Silver was more difficult to extract in this way than gold, but for both metals, the number of contacts necessary to give best results made further investigations desirable.
Reduction of lead and copper from the matte itself appeared to be the most likely avenue. Small scale tests were, therefore, made along these lines, and when a promising result was obtained, experimentation was carried out on a larger scale in the gold-room. Eventually a method using soda ash was evolved that recovered nearly all the gold and silver from secondary matte in a lead-copper base bullion, which, by cupellation, yielded a precious metal bullion suitable for sending to the Mint.
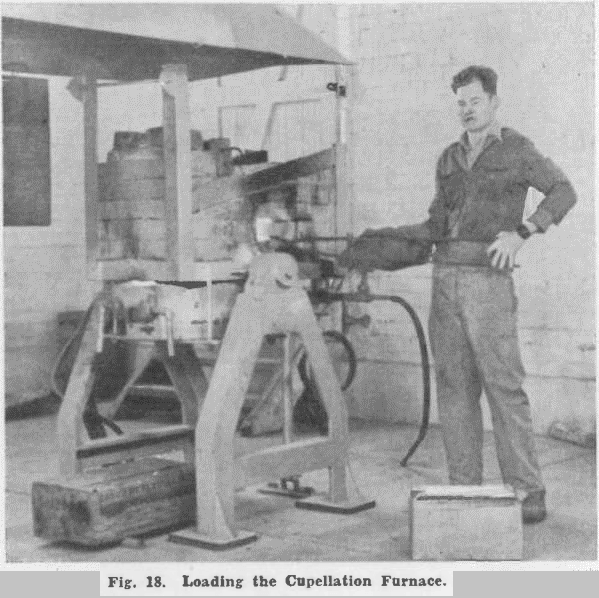
Products from cupellation were then subjected to investigation, and a process was developed for recovering all metals of value from them.
The reduction of secondary matte, with its auxiliary recovery processes, has been adopted as standard gold-room practice since January, 1944, and is likely to be in use until it is possible to smelt treated zinc slimes without production of matte.
In the report that follows, an account is first given of the various products of the matte smelt, in order to familiarise the reader with their composition and characteristics, before proceeding to the investigation work done on the recovery of their metal contents.


By Products of Gold Refinery
Slimes smelted by the matte process before 1944, yielded the following products:
Primary slag:
From the initial smelt.
Primary matte slag:
From the first remelt of primary matte with 20% soda ash.
Secondary matte slag:
From the remelt of secondary matte with 20% soda ash.
Primary matte:
From the initial smelt.
Secondary matte:
By remelting primary matte with 20% soda ash.
Tertiary matte:
By remelting secondary matte with 20 % soda ash.
Fig. 12 makes clear the source of these several products. Two of them, secondary matte slag and tertiary matte, are no longer made, because the new recovery process operates on secondary matte. They will be described, however.
Slags
- Preliminary trials for the recovery of gold from slags included:
- Remelting the slag, with or without added fluxes;
- Grinding followed by straking and cyanidation of the tailings, with amalgamation of the strake concentrates;
- Dry crushing followed by dry-blowing twice, with smelting of the riffle concentrates;
Amalgamation of the whole of the slag in an amalgam barrel, then cyanidation of the resultant finely ground slag. The last method gave most satisfactory results.
Commencing from 7th April, 1943, a complete record of slag treatment has been kept, which includes the assay value of a head sample after each smelt and a calculated head value from the gold recovered by subsequent treatment.
Head values for the period 22nd September, 1943, to 30th May, 1944, are given in Table 2; figures are in oz. per short ton.
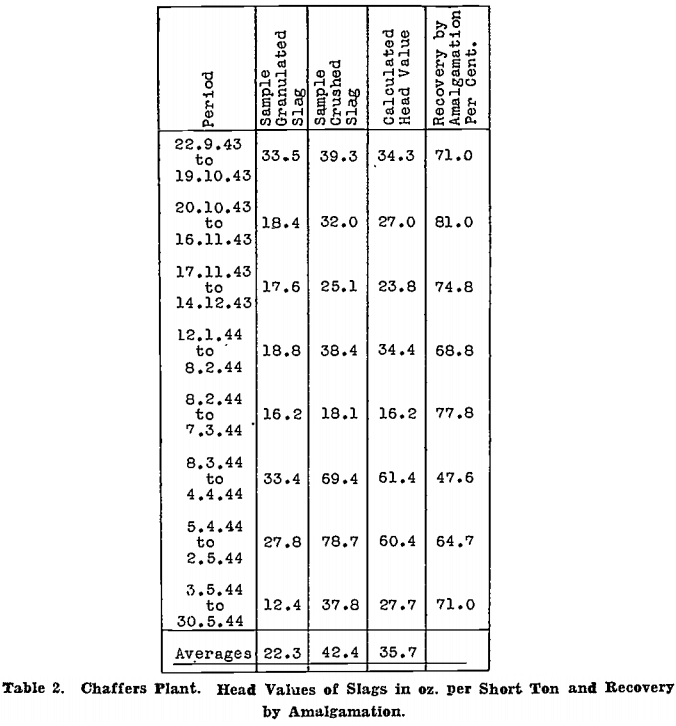
The calculated head value is taken as the most nearly accurate, because it is based on actual gold recovered by amalgamation, and on the assay of finely ground slag, which should contain very little coarse gold, before and after cyanidation. The assays of granulated slag, and of slag dipped from the slag mould while still molten, as shown by previous work not reported here, are quite unreliable. A sample from crushed slag, however, checks fairly closely with the calculated head value and could be used as a guide if the calculated head value from actual treatment were not available.
Table 3 gives figures for the Chaffers Retreatment Plant slags.
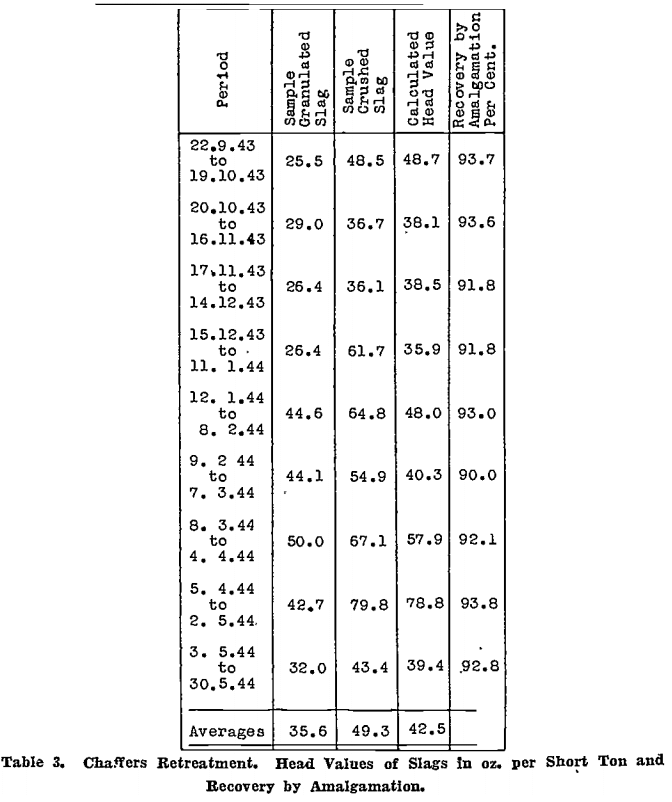
The percentage of gold recovered by amalgamation is given in both tables. The fine oz. of gold in the amalgam is computed with reasonable accuracy from the assay of the composite bar into which it is placed, or if this cannot be done, the retorted gold is melted, poured into a button, and sampled.
After amalgamation, a head sample of the finely ground slag is taken for assay. It is then cyanided in an agitator specifically reserved for this material, for eight days. At the end of this time, a sample is taken, filtered, washed and assayed, if the residue contains 0.5 oz. gold, 8.0 oz. silver per ton, or thereabouts, cyanidation is considered complete and the agitator is discharged through the filtering and precipitation plant with the current roasted concentrates in the main treatment plant.
Cyanide recovery is about 93%, making the overall recovery 99.1% of the gold in the slag from the furnaces. The overall silver recovery from slag is 83%. Chaffers retreatment slags give a recovery by amalgamation of about 92%, with an overall recovery of 99.6%.
Primary matte slag is treated concurrently with primary slag. It contains much less gold and silver than primary slag, and presents no problems when treated by the above method.
- Past Gold Refinery Practice
- Roasting
- Smelting
- Barring Furnace
- Granulation & Sulphuring of Bullion
- Best Gold Refining Methods
- Silver & Gold Smelting Practice
- Amalgamation
- Flotation Strakes
- Roasted Concentrate Strakes
- Retorting of Amalgam
- Removal of Copper from Fine Gold
- Acid Treatment of Fine Gold
- Treatment of Roasted Slime with Sulphuric & Nitric Acids
- Treatment of Raw Slime with Hydrochloric & Sulphuric Acids
- Treatment of Roasted Slime with Hydrochloric Acid
- Treatment with Ferric Sulphate
- Smelting in Furnaces Lined with Carbon-Free Refractories
- Fusion of Gold Slime with Salt Cake
- Conclusion
Mattes
Primary mattes contain roughly 5% of gold and 5% of silver. On remelting with 15% of soda ash, the yield in bullion is approximately 0.6 oz. of bullion per lb. of matte.
Secondary matte carries, on an average, 350 oz. of gold and 2,000 oz. of silver per long ton. Bullion from remelting secondary matte was produced at a rate of approximately 0.6 to 0.7 oz. per lb. of secondary matte. Its grade was very low, and even after granulation and sulphuring with 30% of sulphur, assayed only :—
Gold………………………………………..680 to 560
Silver………………………………………180 to 250
It was, nevertheless, barred without further refining. Matte from sulphuring of secondary matte bullion was treated with the secondary matte for the next month.
The residual tertiary matte, when it was being produced, assayed 30 to 50 oz. of gold and 2,200 oz. silver per long ton.
It will be observed that successive remelts with soda ash reduced the concentration of gold in the matte, but not the silver.
Lead Bath Treatment of Matte
Experimental work, first in the laboratory, using small test quantities, and later in the gold-room, on a plant scale, was carried out by A. C. McDonald, of the Experimental and Research Laboratory, Western Australian School of Mines, in 1943. Extracts from his report (private communication) will be given in this section because of their interest.
The lead bath method was not adopted because work on the reduction of lead and copper from the matte itself was proceeding concurrently with the experimental work by A. C. McDonald, and resulted in a method, involving less smelting, that extracted almost all the silver as well as the gold from the matte. The lead bath method was not successful in this respect.
The programme of work carried out by A. C. McDonald, and summary of results obtained on the lead bath method, follow:—
Programme
“The programme of work in the gold-room was arranged:
- To check the possibility of recovering gold from any one of the several products produced during the smelting treatment of the calcined Merrill slime by remelting any one of these mattes and/or slags and holding it in contact with lead and/or antimonial lead while both are in a liquidus condition, the work to be carried out as a check on the results obtained on the laboratory scale.
- To develop a method whereby a continuity of treatment might be obtained where the resultant gold-lead, and/or gold-antimonial lead bullion would be concentrated to a comparatively high degree prior to cupellation, and at the same time obtain a reject matte sufficiently low in gold value to discard.
- To determine the interdependency of:
(a) Ratio of weight of lead bath and weight of matte treated in a batch treatment.
(b) State of equilibrium of gold values in both the conjugate solutions, i.e., matte and lead bath.
(c) Above (b), with varying gold values in the matte prior to treatment.
(d) Above (b), with varying periods of contact from 15 minutes to 120 minutes.
(e) Above (b), with a varying range of temperatures of the two conjugate solutions.
(f) Other aspects of the process.
Unfortunately, the programme of work was far from being completed at the end of the five-week period.”
Summary
“The preliminary investigation carried out for the recovery of the gold from the matte compounds produced in the Lake View and Star gold-room, indicates a successful treatment for these products.
The laboratory work herein described has shown several interesting features, the main one being the stable property of the matte compounds when heated. It would appear that no sulphur can be removed with ordinary roasting conditions, and furthermore, no sulphur is given off, even when the compound is in a liquidus condition. Possibly only by blowing air through the liquid mass could the sulphur be removed.
From earlier experiences of the writer, it has been found that by bringing a gold-bearing material in contact (when liquid) with a bath of molten lead, antimony, antimony-lead, or copper, the gold transferred from the material to the metal bath. Several features controlled the transfer of the gold, viz.:
(a) Temperature (which in turn controls the degree of liquidity, of the two conjoining liquids).
(b) Time of contact.
(c) Ratio of gold in material and gold in the metal bath, i.e., the degree of gold concentration in the metal bath.
(d) Depth of gold bearing material (liquid condition) in the metal bath. (This has a direct bearing on the time of contact. Tests have been carried out where the liquid gold bearing material was 18 in. thick above the metal bath, but good results were obtained after 16 to 20 hours’ contact.)
The matte compounds were found to have a melting point from 610°C. to 650°C. and were quite liquid at 800°C. Consequently this low melting point is a distinct advantage when applying the lead bath treatment for the recovery of gold and silver from the matte compounds.
Tests were carried out using Salamander crucibles, and the results indicated that very little corrosion took place. The treatment of the secondary mattes should be more favourable in Salamander crucibles since less sodium salts are present. The sodium salts have the tendency to react with the silica from, the crucible mixture to form sodium silicate.
The following tests were carried out to determine the efficiency of the lead bath as a collector for the gold and silver contained in the high value matte compounds, since a considerable amount of work has been carried out by the writer with lower value materials.
The following tests were carried out in the laboratory:
Test No. 2 was made on a tertiary matte; the approximate value of this matte being 19.0 oz. gold per long ton. The sample was submitted to four fusion treatments, being in contact with a new bath of lead for each treatment; that is; as the value decreased after each fusion, the reject matte was held in contact with a quantity of new low value lead.
Subsequent to the first fusion, the gold in the matte had been reduced from 19.0 to 6.2 oz. gold per ton, and the lead bullion concentrated to 30.83 oz. gold per ton. After the fourth fusion the gold in the matte was down to 0.26 oz. gold per ton and the lead contacting this matte contained approximately 1.0 oz. gold per ton.
Test No. 3 was made on a secondary matte with an estimated value of 510.6 oz. gold per ton. The method of using a new lead bath after each fusion was also used in this test and the matte was submitted to three fusions.
After the first fusion the gold in the matte had been reduced from 510.6 oz. gold per ton to 3.77 oz. gold per ton; subsequent to the third fusion the gold in the reject matte was down to 0.14 oz. gold per ton and the lead bullion contacting this matte contained 1.4 oz. gold per ton.
Both these tests were carried out for a 120 minute period of contact.
Further tests were carried out using clay crucibles which in turn showed very little corrosion with repeated usage.
Test No. 4 was made on a secondary matte, making a number of short interval fusions, i.e., eight fusions of 15 minutes, and using new lead for each fusion. After the first fusion the matte had dropped in value from 193.3 oz. gold per ton to 110.7 oz. gold per ton. Each, successive treatment produced a lower value reject matte. The eighth and last treatment gave a reject matte having a gold value of 0.2 oz. gold per ton and the lead in contact with this matte assayed 1.9 oz. gold per ton.
Test No. 5 was similar to the fourth test except that the period of contact was 30 minutes in lieu of the 15 minutes, the matte being submitted to six fusions, and the results indicated that at least one more fusion was required. The gold in the matte was down to 1.9 oz. per ton, with the gold in the lead 16.3 oz. per ton.
From the above series of tests it has been established that the gold concentration in the lead bath for the final clean-up must not exceed a figure of approximately 2 oz. gold .per ton of lead. With this concentration, the gold in the reject matte should be below 0.2 oz. gold, per ton.
Test No. 6 was similar again to the previous test, and after the six fusions of 60 minute periods of contact the final matte assayed 0.9 oz. gold per ton with the lead bullion at 3.3 oz. gold per ton, which further confirms the above feature.
Test No. 7 showed that with the period extended to a 90 minute interval, three fusions were required to reduce the value to 0.5 oz. gold per ton in the reject matte, the lead bullion assaying 3.3 oz. gold per ton.
Test No. 8 was abandoned owing to the crucible cracking and the loss of the sample. It was to have been repeated. The time of contact was for three hours.
Test No. 9 was carried out to determine the extent to which the gold could be concentrated in the lead bullion, and also the collecting property of the high grade lead-gold bullion as a collecting agent. A secondary matte was used in this test and the same bullion used for six fusions with a new sample of secondary matte for each fusion.
The test indicated that as the gold became more concentrated in the lead-gold bullion, so the collecting power of the lead-gold bullion decreased, although with the bullion concentrated up to 1,700 oz. gold per ton, it had the power of collecting approximately 39% of the gold contained in the secondary matte (188.5 oz. gold per ton).
Test No. 10 was carried out on similar lines to the previous test, but a sample of primary matte was used, the estimated value being 1,066 oz. gold per ton.
The result of this test indicated a similar feature to Test No. 9, but with a much higher range of gold concentrations in the bullions. With the bullion concentrated to 15,601.7. oz, gold per ton it had the power to collect 58.3% of the gold from the sample of primary matte.
The last two tests were carried put using storage battery lead, which contains approximately 5% of antimony. At this stage the work was transferred to the gold-room. The antimonial lead recovered from the storage batteries was used for the metal bath.
It may be mentioned that the main consideration in this preliminary investigation was the recovery of the gold, as expeditiously as possible, from the matte compounds, the behaviour of the silver being under observation to a lesser degree. At some future time the copper contained in the matte should receive some attention, whereby it may possibly be removed by a leaching process subsequent to a fusion treatment, the solution yielding copper sulphate suitable for re-use in the treatment plant.
The results of the treatment on a larger scale in the gold-room confirmed the laboratory testing in every respect.
It has been established that the gold contained in the resultant mattes from the process is interdependent with the gold content of the metal bath, i.e., a definite state of gold and silver equilibrium exists between the two conjugate solutions. Possibly the copper enters this phase as well, but the behaviour of the copper was not considered at this stage.
The behaviour of the gold was examined with the least number of variables; consequently in this preliminary work the following features were held as nearly constant as possible, but each in turn requires further examination.
These features were:
- Ratio of weight of metal bath to weight of matte being treated. In all of these tests the ratio at the beginning of the test was one of matte to one of metal.
- Temperature of conjugate solutions. (In the plant testing it was aimed to maintain a temperature of 900°C.)
- Time of contact. (Plant tests were for 90 minutes of contact in most instances.)
- Thickness of layer of top solution (matte) on the metal bath.
The behaviour of the silver and copper subsequent to the removal of the gold also requires investigating with conditions varying as follows:—
(a) Increased time of contact.
(b) Re treatment with a low value metal bath.
(c) Introducing a reducing agent with the charge, e.g., powdered coal, charcoal or sawdust.
(d) Introducing an oxidising reagent with the charge, e.g., manganese dioxide.
(c) and (d) have been suggested since it is fairly evident that the silver is present in solid solution, or as a chemical such as a sulphide or sulphate.
It is interesting to note that in one test an attempt was made to operate the de-golding process on a continuous basis, but the unit proved to be too small. However, an interesting phenomenon showed up with the silver.
The matte compound was one which had been treated on previous occasions, the gold and silver being reduced to 3.0 oz. gold per ton and 322.4 oz. silver per ton.
During the retreatment of a charge, No. 3, of this material, the furnace was using a direct fire, that is, the flame contacting the charge, and a low value metal bath was being used. The values were reduced to 0.05 oz. gold per ton and 22.0 oz. silver per ton.
These results have established a phenomenon which requires further investigation as outlined above, whereby the silver may be released from the matte compounds as well as the gold, and collected by the metal bath.
The following conditions may have had some effect on this result:
- Direct firing of the charge.
- Increased ratio of metal bath to matte compound. (75 lb. lead bullion, 15 lb. matte.)
- Increased time of contact with a low value metal bath. (Fired for a further 60 minutes.)
- Thickness of liquid matte layer conjoining the metal bath, together with one or other of the above features. (In the above test, the layer of 15 lb. matte was not much greater than 5/8 to ¾ in. thickness.)
One other important feature, which may have some bearing on the resultant programme of treatment, is that the final bullion for cupellation should have the gold concentrated to some high figure. Consequently a series of tests was made.
In this set-up, secondary matte added as three separate charges of 100 lb., 100 lb. and 96 lb. to 103 lb. of bullion, gave reject mattes of approximately 20.0 oz. gold per ton and 3.519 oz. silver per ton. This bullion was then used for running down with a primary matte sample. The residual matte from this treatment gave a value of 44.0 oz. gold per ton and 1,496 oz. silver per ton, and the bullion was further concentrated to 2,157.2 oz. gold per ton and 3,862 oz. silver per ton.
These several parcels of reject mattes were then retreated. The results show that subsequent to this treatment the reject matte from all of the above parcels averaged approximately 4.0 oz. gold per ton, whilst the bullion assayed 320.4 oz. gold per ton and 2,412.4 oz. silver per ton.
With the cyclic treatment, this bullion would be re-used with new secondary matte, and the reject matte, i.e., 389 lb. with a value of 4.0 oz. gold per ton, would be retreated a third time with new low value lead.
Of course these results were obtained with one set of conditions. With increased time of contact the gold and silver values may be reduced to much lower figures in the one fusion.
In the gold-room work, an interesting feature to note is the behaviour of the silver. When using 103 lb. of (value unknown) low-gold, silver-lead bullion, the first reject matte assayed 15.5 oz. gold per ton and 1,019.5 oz. silver per ton. The second reject matte assayed 24.4 oz. gold and 1,410.6 oz. silver per ton, and the third reject matte assayed 21.6 oz. gold and 1,763.4 oz. silver per ton.
The interesting feature of this test is that, although the gold content of the bullion increased to 1,161.0 oz. gold per ton, the gold in each of the reject mattes remained at about the same 20.0 oz. mark, although the silver value increased progressively in the reject mattes, e.g., as the silver concentrated in the bullion from an unknown figure to 2,981.8 oz. to 3,591.0 oz. silver per ton, the silver in the corresponding mattes varied from 1,019.5 oz. to 1,410.6 oz., to 1,763.4 oz. silver per ton.
A further interesting feature shows up in the second treatment of these reject matte compounds in this work. In this treatment new lead was used for the first fusion, the resultant bullion from each fusion being used for the next. The values showed up as follows:—
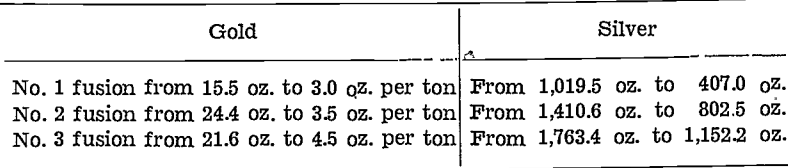
The above shows that the collecting power of the bullion for the silver was fairly constant, although the bullion progressively became concentrated with both gold and silver. The amount of silver removed during each fusion was approximately 610 oz. silver per ton.
Since the first fusion was made with 87 lb. lead and 76 lb. matte, and 612 oz. silver per ton was collected, it may be reasonable to assume that the 600 oz. silver per ton is the amount that can be collected during the 90- minute time of contact. Further tests should be made with increased periods of contact.”
Reduction of Mattes to Metals
Although the lead bath method showed promise of good gold recoveries from the matte, given sufficient time to contact in a cyclic treatment, using low gold-value lead in the last cycle, the method did not accomplish the chief objective, namely, the recovery of all the metal values economically.
Reduction of Matte to Base Bullion
Reduction of lead from the matte itself appeared to be the most likely avenue for further investigation. A series of tests was therefore made, adding several different reducing agents to a matte charge to produce a fall of lead.
Preliminary tests on a small scale were made on secondary and tertiary mattes to determine the best starting point for any process developed. On the results, secondary matte appeared the better of the two for plant practice, but there was an accumulation of tertiary matte already on hand that had also to be satisfactorily treated. Consequently a set of laboratory tests on each type of matte was made.
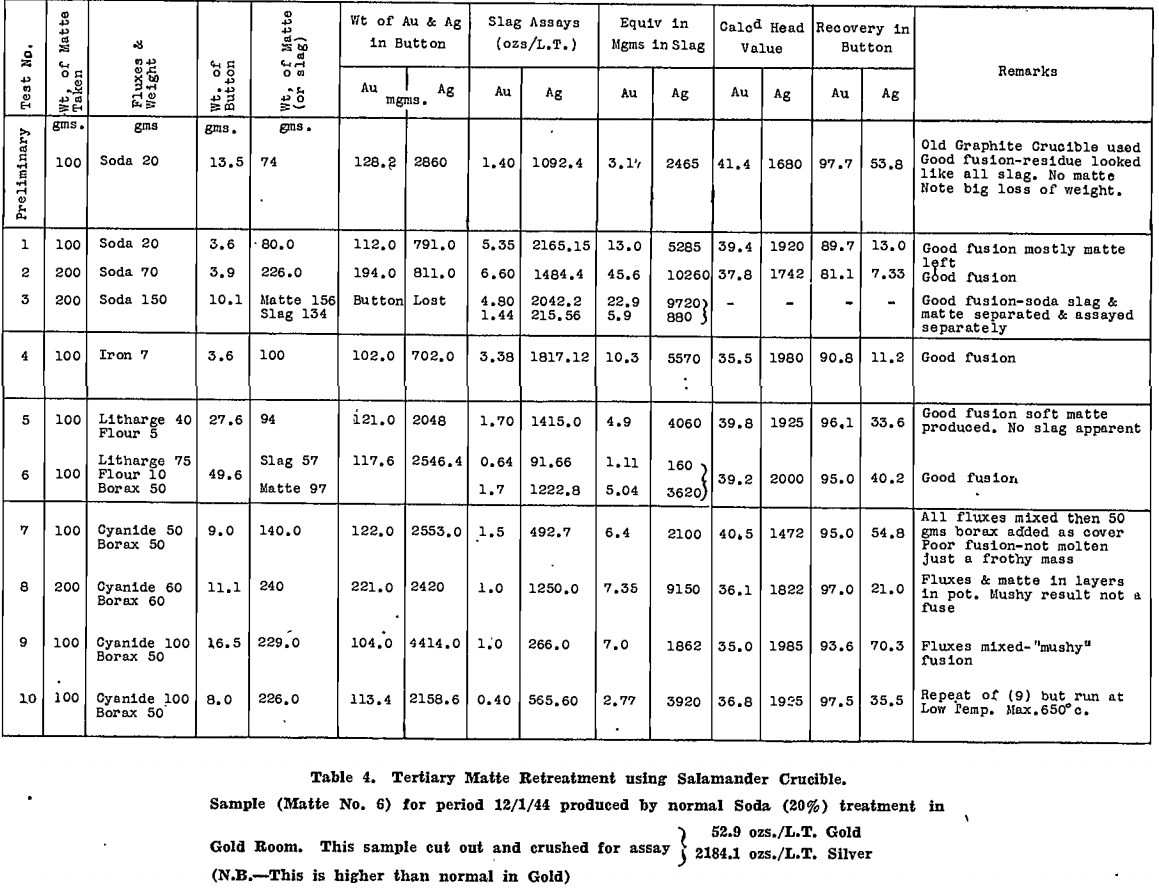
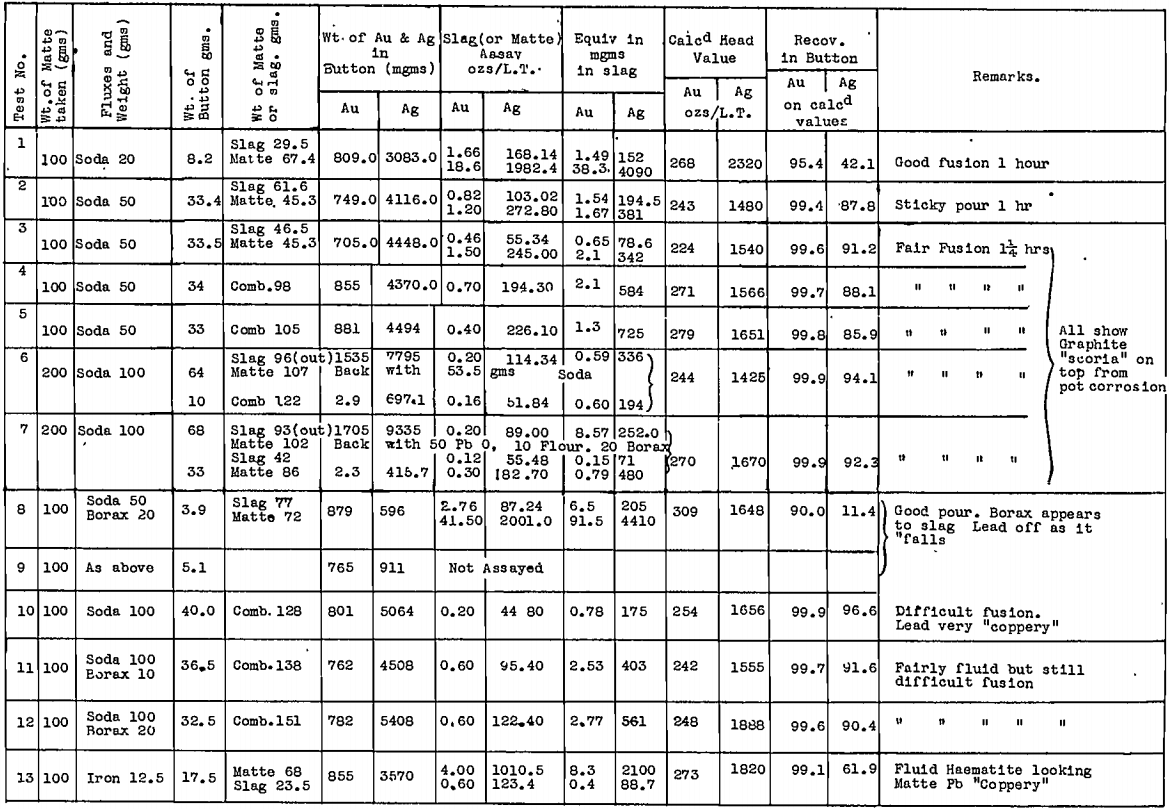
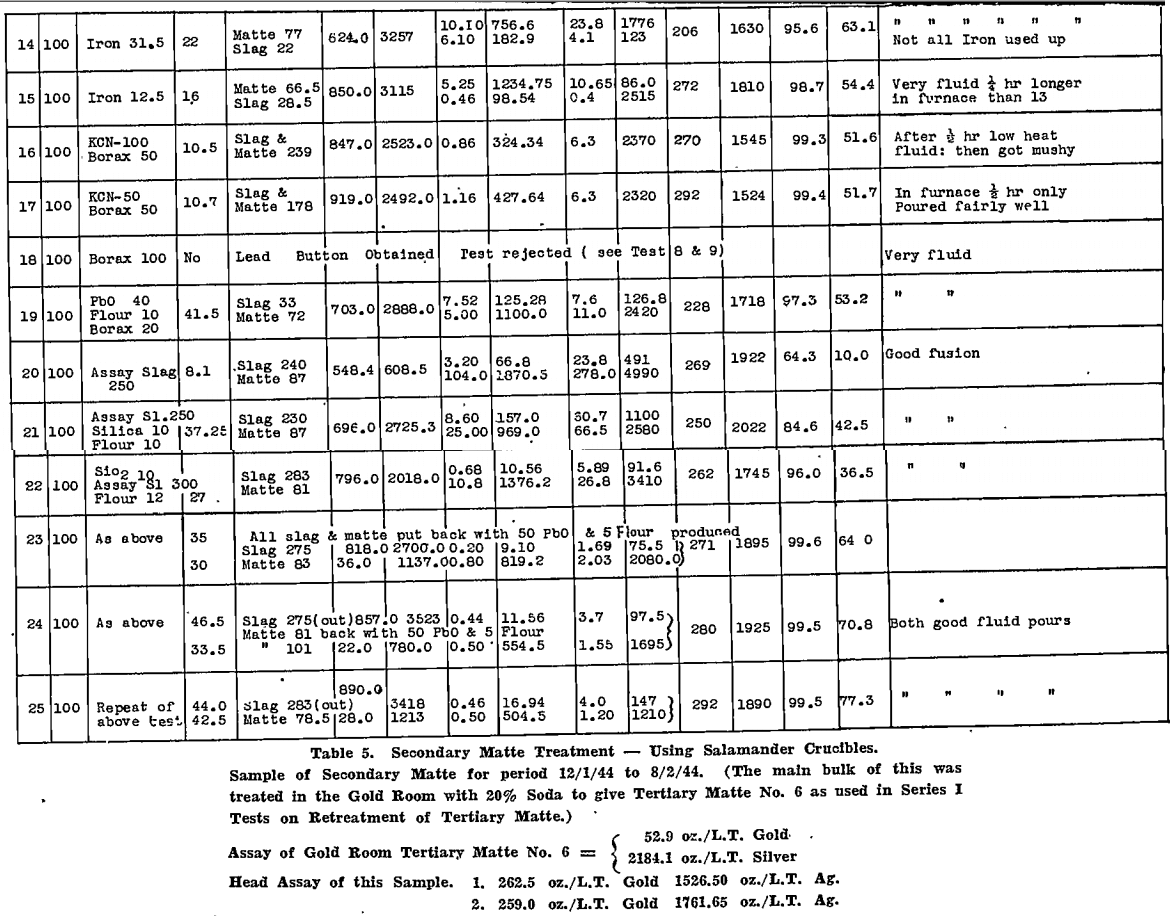
All tests were carried out in Salamander crucibles so that large scale tests in the gold-room, using No. 9 Salamander retorts or silicon-carbide lined Wabi furnaces, could be expected to follow the small scale tests closely. The results are tabulated for secondary matte in Table 4 and for tertiary matte in Table 5.
Deductions made from these tests are summarised as follows:
- Melting the matte with 50% by weight of soda ash, with added reducing agent, gave very good recoveries of gold and silver in the base bullion produced, particularly with secondary matte.
- Time of contact with the soda, after fusion was complete, was important. This was appreciated only after a considerable number of tests had been made. Insufficient time after fusion accounts for the poor silver recoveries reported in the tests on tertiary matte, which were conducted first.
- In all tests where the silver recovery was good, a large fall of base bullion was recorded, whereas gold recoveries were not affected so much by a reduction in bullion weight.
4. In certain tests where a large lead fall was produced, by using litharge or assay slag, the silver recoveries were not high.
5. On the other hand, those fluxes that produced a large metal fall from the matte itself, gave the best results, particularly when the bullion produced was coppery in nature.
6. Silver was held closely by the copper sulphide portion of the matte.
Some confirmation of (6) was found in an article by Fulton and Goodner on copper-iron and copper-iron-lead mattes. In their conclusions the authors state:—
“Cuprous sulphide and silver sulphide form an unbroken series of solid solutions and there is little doubt that when cuprous sulphide is present, silver is in solution in this substance as sulphide. It is also a powerful solvent for gold.
Metallic copper, usually present in copper mattes, had a powerful solvent action on both gold and silver. Lead sulphide has a considerable solvent action on silver sulphide but only a limited one on gold. Zinc sulphide has very little solvent action on silver or gold. Metallic iron has a powerful solvent action on gold but only a limited one on silver. Ferrous sulphide has practically no solvent action on silver sulphide or gold.”
Confirmatory tests on a larger scale were next made in the gold-room. Table 6 summarises the results of the first 12 of these tests, which will now be discussed in more detail than the table permits.
Test No. 1 was a preliminary test done in a Wabi furnace with Carbolox lining and before the effect of a long contact with soda had been recognised as essential. As will be seen from Table 6, the time of contact was only 1¼ hours, and the recoveries were poor.
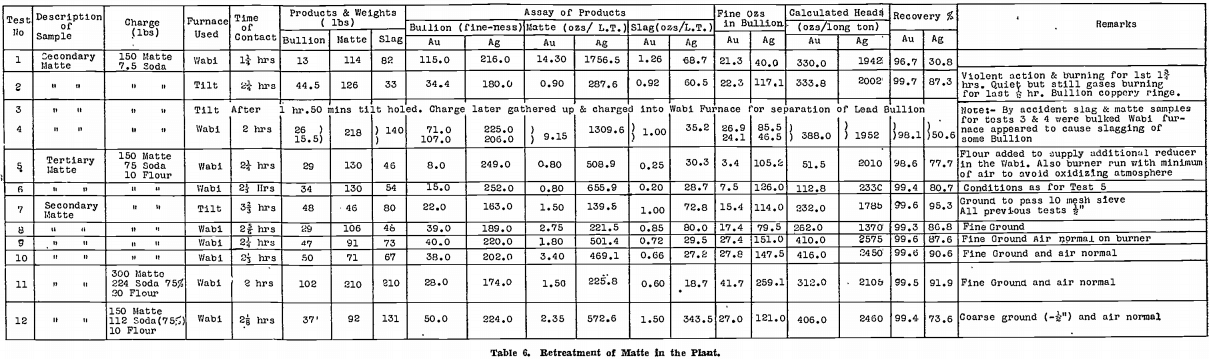
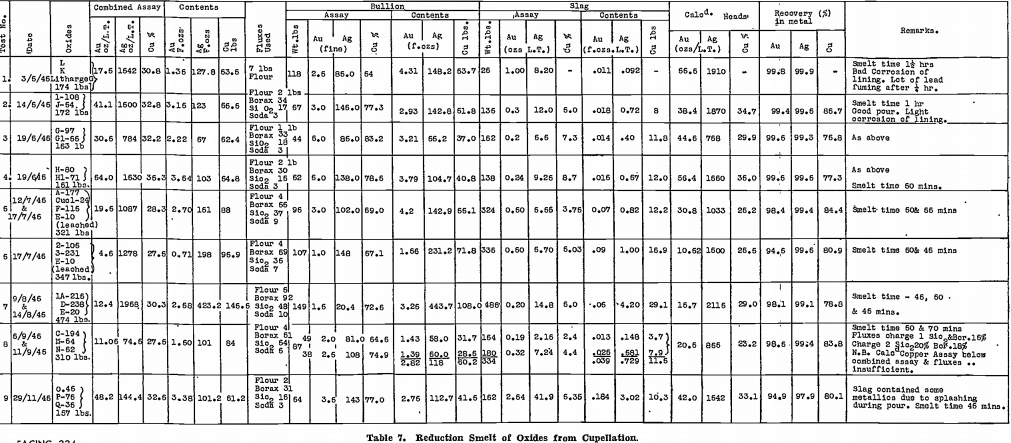
Test No. 2 was run in the Faber du Faur furnace with a Salamander No. 9 retort. This test, with the long contact of 2¼ hours, gave a result as good as any small scale test using the same fluxes. The charge could be watched. There was a violent evolution of gas, with much burning of the evolved gas, for the first 1¾ hours. The charge then became tranquil, but flaming from above the molten mass continued.
The bullion plug was distinctly coppery in colour.
All products were allowed to freeze after pouring, to ensure separation of the matte, slag, and bullion.
Test No. 3 was a repeat of Test No. 2, but after 1 hour 50 minutes the retort holed on the slag line. The charge was held until the time of contact was 2¼ hours and then poured. During the pouring operation the neck holed and practically the total contents of the charge ran through to the well below the furnace. Next day, this was cleaned up and a sheet of lead bullion was observed. The whole melt was recharged into a Wabi furnace in order to separate the bullion. Unfortunately the large sheet of lead which was recovered from the well was not roughly weighed before feeding into the Wabi furnace. Only 26 lb. of lead bullion was recovered on remelting, yet it was considered certain that there was more than this weight to commence with. This led to doubts as to the usefulness of a Wabi furnace in which the flame is in contact with the charge during a reducing smelt.
Test No. 4 was run simultaneously to Test No. 3, in another Wabi. Despite a 2 hour contact, only 15½ lb. of lead bullion was obtained.
At this stage, it appeared as if oxidising conditions prevailed in the Wabi furnace, inspite of the carbide lining. From the point of view of wear on the liner, however, the furnace was quite satisfactory; with the Salamander retorts, wear was excessive!
Tests Nos. 5 and 6. — By this time, all secondary matte on hand had been smelted, and it was decided to carry on further tests with accumulated tertiary matte. The sample taken assayed 52 oz. gold, 2,180 oz. silver per long ton.
Air was cut back to the minimum necessary for complete combustion of the oil, to avoid the oxidising conditions that appeared to be present in Tests Nos. 2 and 3. Ten pounds of flour was also added to the charge to give extra reducing power. Melting of the charge with the reduced amount of air was slow, and the lead fall was not as high as expected.
Tests Nos. 7 and 8 were later run on secondary matte, still using the reduced air on the Wabi furnace, but with normal burning on the tilt furnace. The lead fall in the Wabi furnace was again disappointing, in spite of the longer time of contact, using finely ground material. Results in the tilt furnace, on the other hand, were excellent.
Tests Nos. 9 and 10. — Air was restored to normal for Tests Nos. 9 and 10, conducted in Wabi furnaces, using finely ground material, with 10 lb. of added flour. A good lead fall was obtained, and lining corrosion was small.
This type of smelt at last appeared satisfactory.
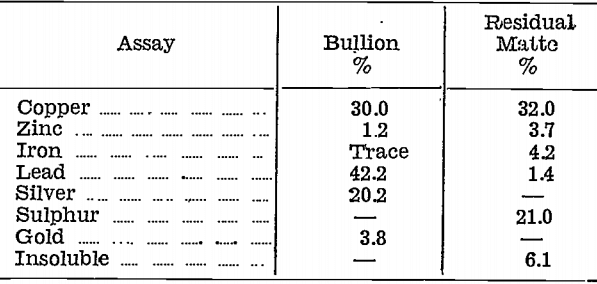
The residual matte and the lead bullion from Test No. 10 were assayed for the base and precious metal contents. Results were as follows:—
Test No. 11 was next run using 75% of soda ash, the matte being again finely ground. The silver recovery was slightly higher than in Tests Nos. 9 and 10. All three of the products were assayed:—
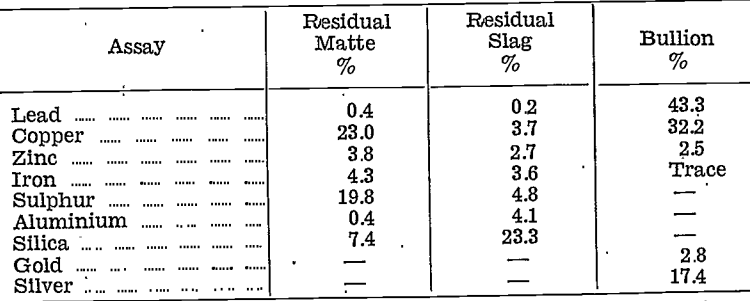
The matte and slag contained considerable amounts of sodium salts, including the sulphide which was plainly noticeable by its odour. Water soluble tests were conducted and the matte contained 41.5% of water soluble salts and the slag 49.7%.
Test No. 12. — Recoveries were adversely affected, although 75% of soda ash was used, by not grinding the matte finely before smelting.
Little improvement had been shown in the two tests succeeding Tests Nos. 9 and 10. Accordingly, that procedure was transferred to the gold-room without alteration, that is, secondary matte was finely ground, mixed with 50% of soda ash and 7% of flour, and smelted for 2¼ hours in the Wabi furnace.
Results confirmed the laboratory tests. Nearly all the gold and silver was concentrated in a base bullion, which, when allowed to cool overnight, could be readily separated from the residual matte and slag. Furthermore, the slag layer could be identified and separated from the residual matte layer.
The amount of copper in the base bullion is important in two respects. Firstly, unless 20% or more is present, it is found that silver is not satisfactorily removed from the matte; secondly, an excess of copper up to 50% makes subsequent cupellation difficult. It has not been found necessary, however, to attempt the control of the copper content in the base bullion beyond the practice stated above.
Secondary matte is accumulated for one month before crushing and smelting, about 300 lb. yielding 70 lb., approximately, of base bullion. This is broken under a power hammer into pieces of a size convenient for inserting with a pair of tongs into the cupelling furnace.
Slag is of no value and is dumped. Residual matte contains between 20 and 40% copper, 1.5 to 2 oz. per ton gold, 100 to 200 oz. per ton silver, and is stacked in the open to weather for approximately six months. It is then washed four times by decantation with water, using a pulp ratio of about 1.5 water to 1 matte each time. Loss of weight on washing varies from 51% to 30% of original weight, so that the residue contains about 3 oz. gold, 300 oz. silver per ton.
The residues have not yet been further treated, but it is expected that by smelting it will be possible to recover the gold, silver, and copper as a copper base bullion, which can be sent to a smelter. In that case, the copper should pay for all shipment and smelting charges.
The value of gold and silver tied up in this, way in the residual matte is very small, as shown in the summary of distribution of values given in Fig. 14, and for all practical purposes, the secondary matte may be considered to be cleaned of all payable metals by the reduction smelting process just described.
Cupellation of Base Bullion
A cupellation furnace was built to refine the base bullion produced in both the lead bath and the reduction processes for secondary matte treatment. Perhaps the most important feature of this furnace is the refractory lining used to form the cupel test. It was developed by A. C. McDonald in earlier work on a larger scale, and was the only material found to resist satisfactorily the intense slagging action of the oxides formed. A description is therefore given here of its composition and method of forming the test.
Refractory Lining for Cupel Test
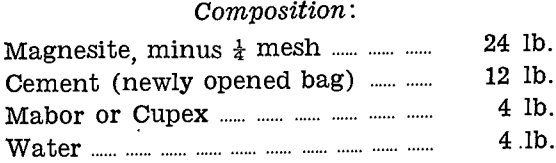
Mixing:
The three constituents of the charge are thoroughly mixed dry by completely turning over at least six times. The well mixed dry charge is then spread on a clean plate to a uniform depth, not greater than 3 in., and the water added through a watering can rose over the whole area. It is then well turned until no dry portion remains.
Tamping:
All-the mixed material is shovelled immediately after mixing into the cupel basket and levelled out, so as to contour the cast iron pan, the bed at this stage being about 3½ to 4 in. thick.
A 4 lb. gympie hammer with a short handle is used for tamping, commencing in the centre and working outwards. Force of the blows with the hammer is increased as tamping proceeds, until no further impression is made, when the 4 lb. gympie is replaced with a 2 lb. mason’s gympie. Tamping is then done more carefully with greater force, each hammer mark being partly covered by the subsequent blow.
When tamping has been completed, the surface is finished off with a trowel. Usually sufficient water is
present to float some mabor to the surface for this phase, but if not, a little can be sprayed on with a bannister brush. The mabor fills any interstices in the surface and produces a very smooth finish. The lip over which oxides are poured is formed during tamping and trowelled at the same time as the rest of the test.
Thickness of the test on completion is about 2 in.
Time taken for the whole operation is about four hours.
Tests have been successfully used after standing only 20 days, but as a test lasts about three to four months, the spare usually has had much longer than the minimum time to cure.
Treatment of old Furnace Liners
When a test cracks it is replaced with the spare, and the old lining is crushed in a small dry ball mill that is on hand for such work in the goldroom. The crushed material is disposed of by feeding into the reduction smelt of secondary matte, at the rate of 10 lb. lining per 150 lb. charge of matte.
Cupellation
Base bullion from the secondary matte reduction smelt is assayed for silver and copper before cupellation. The course cupellation will take is guided according to the percentage of copper present. If this is low, cupellation can be conducted at a faster rate. at a lower temperature than if the copper percentage is high.
Lighting the cupellation furnace differs from usual practice in that a few sticks of pine wood only are used to ignite the oil. It will be remembered that with all the other furnaces, the practice is to have a good wood fire burning for ¾ hour or more before turning on the oil.
With oil on, the furnace is brought up to a red heat as slowly as possible to prevent cracking the cupel test. Preparatory heating takes about 1½ hours.
Pieces of matte bullion, 40 to 50 lb., up to a maximum of 100 lb., are loaded into the cupel through the side door. Preheating the cold bullion, either outside, or within the furnace, before allowing it to come in contact with the lining, has not been found necessary.
During melting, metal runs in droplets away from each piece of bullion, until at one stage, lumps are left that are rather difficult to melt, particularly with high-copper base bullion. They are stirred in the molten metal with a bar until all is molten.
Air is next played on the surface through an adjustable ½ in. pipe. The pipe is directed to a point about 2 in. from the metal line at the burner end, and tilted slightly forward. The amount of air used is sufficient to cause a decided ripple in the surface of the molten metal, but not enough to cause splashing.
An oxide layer forms on the surface, and is allowed to grow until the metal is covered to a depth of about ¼ in., or until the “eye” below the air jet is just showing. Oxides are then poured off in a thin stream over the lip by tilting the furnace forward slowly. They are granulated directly by falling into a four gallon kerosene tin full of water.
The oxides overflowing the lip are tested at frequent intervals by collecting some on an ordinary dry shovel, moving the shovel while the stream falls on it to give a thin layer of material. Metal adheres to the shovel and, unlike oxide, is not readily dislodged by shaking up and down. Another indication is by observation of the stream. Oxides rarely run straight over the lip, but follow an uneven line, whilst metal falls in a straight stream. If metal should appear on the shovel or the eye of metal in the cupel below the air jet gets too large, the furnace is tilted back slightly to stop the flow until more oxide has accumulated, when pouring is recommenced.
Cupellation ceases fairly abruptly, a few circles of litharge race about on the surface and are soon absorbed by the liner, leaving a brilliant metal surface.
The refined bullion is finally poured into a conical mould. When it has frozen, the button is tipped on the floor, quenched in water, and is then ready for barring.
Recovery from Cupellation Oxides
The simple cupellation procedure of current practice was preceded by much experimental work, because, although a good grade of precious metal bullion could be readily made, there remained the problem of recovering gold, silver, and copper from the cupellation oxides.
Recently it has been found that by analysing the oxides and calculating a flux to form a sesquisilicate slag with the lead only, it is possible to effect an almost complete separation of copper, gold, and silver in a copper base bullion from a lead silicate slag, by a reducing smelt, again a relatively simple process.
Preliminary Test Work: Before the reduction of a copper base bullion and the slagging off of the lead was adopted, a number of methods was tried on both laboratory and plant scales for the recovery of valuable contents. A description of these is now given, ending with the laboratory scale tests which led to the adoption of the reduction smelt to a copper bullion as standard plant practice.
When secondary matte bullion first came forward for cupellation from the reduction smelt of secondary matte, little was known of the course cupellation would take, although it was expected that with a high percentage of copper present in the bullion it might be difficult to cupel without the addition of sufficient new lead to lower the copper percentage to 15%.
Nevertheless, a trial cupellation was made on matte bullion alone, and by increasing the temperature as cupellation proceeded, bullion of the following grade was produced:—
Gold……………………………………….141
Silver……………………………………..813
Base metal………………………………46
During the first cupellation, it was observed that a marked physical change occurred at a point about midway through the cupellation. Differential oxidation was suspected to be the cause, and in the next cupellation, samples of the oxides formed were taken for assay early, midway, and late in the cupellation. Assay results confirmed that lead was being oxidised sooner than the copper:—
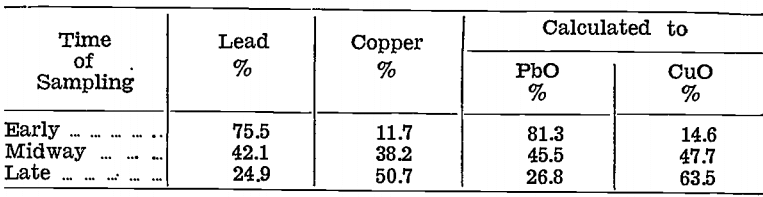
Gold and silver were distributed unevenly in the impure litharge and copper oxide stages, as will be seen from the following table, which shows the amounts of the various products with their contents for the first 548 lb. of matte bullion cupelled:—
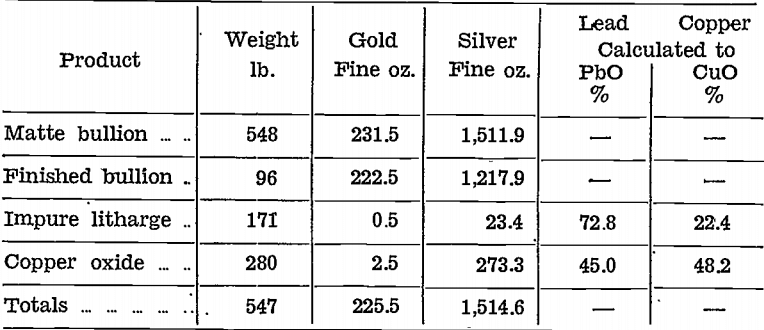
Neither product could be rejected because both contained precious metals and, copper.
Further investigation work on the cupellation oxides was conducted with three main objectives:—
- Recovery of the gold and silver.
- Recovery of copper in a useful form.
- Recovery of sufficient lead of low copper content for subsequent cupellations. Lead, in itself, was not considered worth recovering as a commercial product.
Impure litharge, when reduced, satisfied the last condition, the lead also collecting all the gold and silver. Copper content of the lead so reduced, was higher than hoped for, but no further work was done on this product.
The copper oxide product was treated by partial reduction only, when it was found that copper reduced before lead. Reduction was then carried out in two stages, first by adding sufficient reducer to convert 10% of the original weight to metal, second by completely reducing the residue. The compositions, in percentages, of the two metals obtained were as follows:—
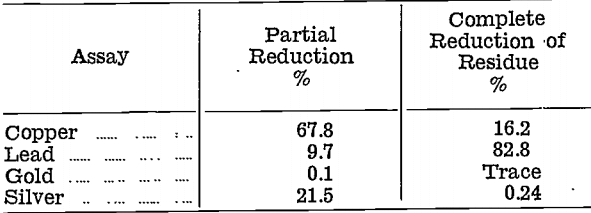
Coppery metal from the partial reduction, with its high content of gold and silver, was considered too difficult to treat, and the method was abandoned.
Next, a complete reduction was made. Sulphur was stirred into the resulting metal to remove copper as a matte, and the two products were assayed:—
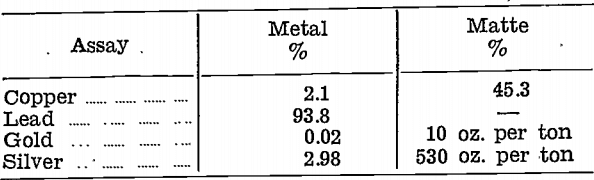
This treatment method was also rejected, for two reasons:—
- The lead, although suitable for breaking down the copper concentration before cupellation by addition to matte bullion, was produced in too great a quantity.
- High values in the matte would necessitate returning it to the secondary matte smelt, thereby producing a closed copper circuit.
Liquation of the completely reduced metal was attempted without success. Liquation of the oxides was also considered as a possibility before any reductions were made, but separations were very incomplete, and the extremely corrosive properties of the molten oxides presented some difficult equipment problems.
Leaching with sulphuric acid to remove the copper was next tried. The product was ground to minus 35 mesh and leached with sulphuric acid of 5% maximum strength. Copper recovery, after 48 hours, was 72.3% as copper sulphate. Finer grinding increased the speed of copper dissolution, but recovery rose only slightly.
The resulting copper sulphate solution was evaporated and allowed to crystallise. Allowing costs for grinding, acid consumption and power, crystallised copper sulphate could be produced for less than the existing market price. The yield would be approximately 16 cwt. of copper sulphate annually.
The residue, after leaching, was much finer than the original feed, as disintegration took place during leaching. The residue was next partially reduced to give a 30% by weight fall of metal. Lead so produced contained 7.4% of copper. The residue carried only 0.20 oz. gold and 8.4 oz. silver per ton. Therefore, it was considered that if the copper leach could be improved sufficiently to give a lead lower in copper, the three objectives of the investigation would be attained.
The next cupellation was run, using 40% of new lead, reduced from the litharge of the previous cupellation. Although the cupellation was much more easily controlled after this addition, a copper oxide product was still produced in the later stages. Bearing in mind the results of the foregoing leaching tests, it was decided not to separate the two stages, but to granulate all the oxides into one bulk lot.
Details of this cupellation, showing the weights and distribution of values are given in the table below:—
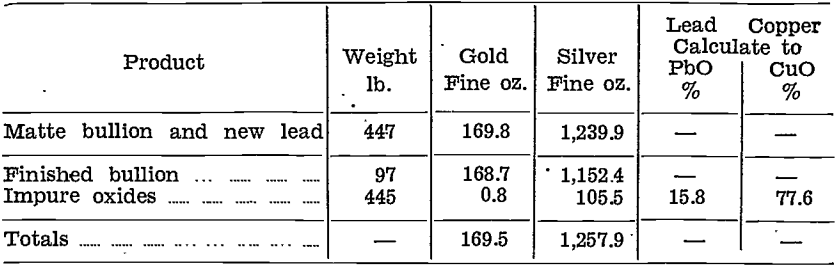
Leaching tests on these impure oxides were made, and were quite successful. Recovery of copper as sulphate was no higher than with the copper oxide product described earlier; on the other hand, lead reduced from the leached residue contained only 4.9% of copper. This was because of the lower percentage of copper in the original oxides.
Gold and silver recoveries into the lead were almost complete.
Leaching on a plant scale was not as successful as the small scale tests. An unexpected complication appeared after the first batch had been leached twice. On adding the third wash of dilute sulphuric acid, a metallic copper film formed over the oxide particles and dissolution of copper ceased.
Smelting tests were then made on a laboratory scale in an attempt to slag off the litharge, from the cupellation oxides, at the same time reducing the copper oxide to metal, which would act as a collector for gold and silver. The quantities and kind of flux used were empirical, until preliminary tests had shown that borax, with charcoal as reducing agent, gave the best results. To reduce the cost, borax was then partially replaced with an equivalent amount of silica to give a boro-silicate slag.
Finally, it was found possible to calculate the flux required from the analysis of the oxides. This is now done by calculating the amount of silica necessary to form a sesquisilicate slag, then replacing silica by its equivalent in borax, so that the final flux contains two parts borax to one part silica.
For example: Suppose a charge requires 30 lb. silica alone to form a sesquisilicate slag.
Let x be the amount of silica required.
Then 1.5 (30 – x) is the amount of borax required, because 1.5 borax is equivalent to 1 silica in slagging action.
But as the amount of borax is to be twice the amount of silica in the final flux,
Therefore 1.5 (30 – x) = 2 x .
Whence x = 12.9 approximately
=13 approximately
The flux would then be:—
Silica……………………………………….13 lb.
Borax………………………………………26 lb.
Soda ash, equal to 2% of the weight of oxides is added. No allowance is made for this in calculating the silica-borax flux. Its effect is not only to increase fluidity, but to hasten melting, so that the charge is in contact with the reducing carbide liner of the furnace for the minimum time.
Flour, 1.33% of the weight of oxides being taken, is added as the reducing agent.
A peculiar effect is noticed when pouring this particular charge. When the slag enters the large conical mould, there appears to be a large evolution of gas, and the whole charge froths up, filling the mould. It subsides again after a few minutes to a normal-looking slag of a deep chocolate colour.
Table 7 gives details of plant tests conducted on the cupellation oxides in a Wabi furnace, using the method of reduction smelting described above. It will be noted from the figures that the recovery of gold and silver into the copper base bullion is almost complete. Recovery of copper is not as high, some being lost into the slag, but its value is slight.
Bars of copper base bullion produced to date, total 16 in number. They have been sampled in groups for assay, the results and weights being shown in Table 8. Other base metals, not analysed, mean principally lead, which has been reduced during the smelt, and carries a penalty when the bullion is treated by a copper smelter. Again, its negative value is low, so that when this copper base bullion is eventually shipped, the return should be approximately the full value of the gold and silver contents, the copper paying freight, smelting charges, and penalty for lead content.
Summary and Flow Sheet
Gold and silver are recovered successfully from secondary matte by a series of smelting operations set out diagrammatically in Fig. 13. Fig. 14 shows the gold, silver and copper distribution in the various stages.
Taken in order, the separate treatment processes are:
- Secondary matte is ground in a dry ball mill before mixing with 50 % of soda ash and 7 % of its weight of flour. Charges containing 150 lb. of matte at a time are smelted for 2¼ hours in Wabi, silicon-carbide lined furnaces, yielding three products, residual slag, residual matte, and a copper- lead base bullion that collects nearly all the gold and silver, with about half the copper from the secondary matte.
- The lead-copper base bullion is cupelled in a special furnace, producing high grade gold-silver bullion suitable for shipment to the Royal Mint, and a mixture of cupellation oxides which still retain small amounts of gold and silver, that are, nevertheless, worth recovering by further treatment.
- Cupellation oxides are smelted at intervals, using a calculated silica-borax flux with a small percentage of flour added as a reducing agent. Copper base bullion produced by this smelt contains the remnant of gold and silver in the
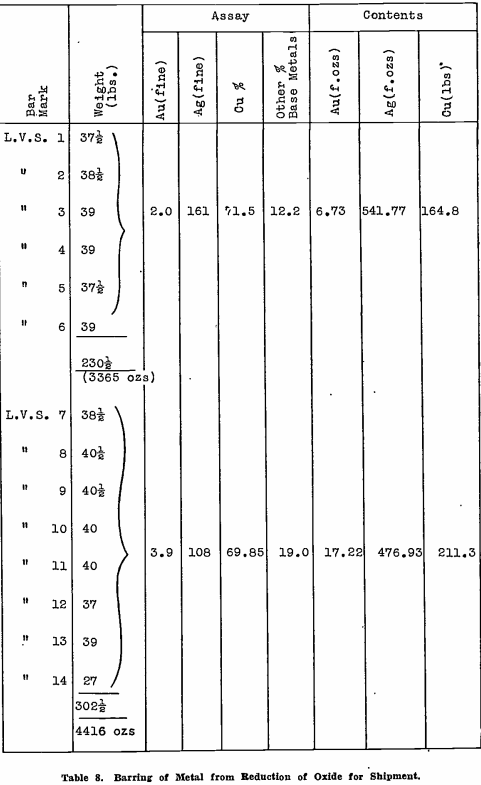
cupellation oxides, as well as most of the copper, with only a small percentage of lead, so that the precious metal values can be recovered at low cost by shipment to a copper smelter.
- Gold and silver retained by a cupel test is recovered, after the test is of no further use for cupellation, by grinding and feeding back to the reduction smelt of secondary matte at the rate of 10 lb. per 150 lb. charge of matte.
- There are two by-products only — residual matte and residual slag — the former of which still retains gold and silver in sufficient concentration to consider further treatment; even so, the quantity of these materials produced is very small.
- The original objective of the investigation, namely the recovery on the mine of all gold and silver values from mattes and slags, has been practically achieved.
