The U. S. Bureau of Mines has been investigating the application of thiosulfate to recover gold directly from low grade refractory carbonaceous ores for the past four years. Statistical experimental methods were used to determine the effects of various leaching parameters on both gold extraction and thiosulfate consumption. Seven variables were investigated, including thiosulfate, copper, ammonia, sulfite, and sulfate concentrations, leaching time, and leaching atmosphere. This information was used in face-centered cubic (FCC) surface response experiments to define the optimum leaching conditions for a low grade ore. Two models were generated from the data to predict gold extraction and thiosulfate consumption as a function of the leaching parameters. The models predicted a gold extraction of 67 pct and reagent consumption of 2 kg S2O3²-/mt ore at 0.25 M S2O3²-, 0.0014 M Cu²+, 0.27 M NH4OH, 0.00625 M SO3²-, and leaching for 16 h. Actual tests resulted in 62 pct gold extraction with 0.93 kg S2O3²- consumed/mt ore. Subsequent column leaching tests have further demonstrated the viability of applying this technology to recover gold from carbonaceous ores without first pretreating the material. Gold extractions in the column tests ranged from 69 to 78 pct with thiosulfate consumption ranging from 0.4 to 5.4 kg/mt ore. These results compare to <5 pct gold extraction using standard cyanide leaching conditions.
Gold ores can be predominantly classified into two general categories based on the host material: refractory and non-refractory. While the non-refractory ores (typically oxidized ores) can be processed for their precious metal values using standard cyanidation methods, the refractory ores (such as sulfidic and carbonaceous) require special techniques for recovering the values.
Ores containing sulfides are generally refractory due to encapsulation of the gold and silver by sulfide minerals. In this type of system, the sulfides must first be destroyed before the precious metals can be recovered using cyanide. Ores containing carbonaceous material are somewhat different. Typically the gold is dissolved by cyanide, but the gold cyanide complex is immediately adsorbed by the carbonaceous material in the ore before the gold can be recovered from solution. This is commonly known as “preg-robbing”. Various methods have been suggested and used on a commercial scale for treating both of these types of ores.
Autoclave pre-oxidation is a common method used to treat sulfide ores, but has only recently been applied successfully to carbonaceous ores (Simmons, 1995). Roasting is the preferred method for carbonaceous ores, especially when sulfides are also present. Alkaline chlorine pre-oxidation of the carbonaceous material was patented and also tested in a pilot plant by the USBM in 1972 (Scheiner, et al., 1972a, 1972b) and has also been used on a commercial scale (Guay, et al., 1973), but special precautions must be taken in order to minimize the extent of corrosion on operating equipment. Blinding of the carbonaceous material with organic compounds prior to cyanide leaching has also been attempted as a method to minimize the adsorption of gold cyanide onto the carbonaceous material. Another method which is used in stan¬dard cyanidation circuits is carbon-in-leach (CIL) or carbon-in-pulp (CIP), where the activated carbon competes with the carbonaceous material for the soluble gold cyanide complex. Normally this method is only used in pre-existing milling circuits already using cyanide in an attempt to increase the recovery of gold.
These methods are only useful for high grade ores, where the ore is finely ground, or contains enough values to support a pretreatment stage. Low grade ores do not contain enough values to justify this type of processing and are therefore typically classified as waste. This suggests that a direct treatment method should be developed to make these ores economical to process.
Work conducted by Gallagher, et al. in 1990 showed that the gold thiosulfate complex did not readily adsorb onto activated carbon, suggesting that thiosulfate may be a suitable lixiviant for ore containing carbonaceous material. Hemmati, et al (1989) proved that the thiosulfate leaching system could be used to recover gold directly from high grade refractory carbonaceous ores. Much higher recoveries were obtained when using thiosulfate as a lixiviant instead of cyanide, however reagent consumption was high due to the extreme leach conditions used. Work conducted by the USBM (Langhans, et al., 1992) showed that thiosulfate could also be applied to low grade ores. When using the proper leach conditions, comparable gold recoveries and competitive reagent consumption were realized compared to cyanide. This prior work suggested that it should be feasible to leach gold directly from low grade carbonaceous ore using thiosulfate. Indeed, Newmont recently applied for and received a patent covering this technology (Wan, et al., 1994). Several papers (Li, et al., 1995, Wan, et al., 1993) have been written to provide valuable insight into the thiosulfate system, but no details of Newmont’s process have been published yet.
This paper describes work conducted by the U. S. Bureau of Mines to develop a direct method to recover gold and
silver from low grade refractory carbonaceous ores. Information is provided as to the leach conditions appropriate for a heap, as well as pertinent results. Research focused on maximizing gold extraction while minimizing reagent consumption at conditions suitable for heap leaching.
Experimental Procedure
Thiosulfate leaching studies were conducted on a low-grade refractory carbonaceous ore from northeast Nevada. Analysis of the -100 mesh ore is given in Table 1. X-ray diffraction indicated major quartz (SiO2) with a minor to trace amount of mica closest to muscovite (KAl2(Si3,Al)O10(OH,F)2) and trace amounts of dolomite (CaMg(CO3)2), pyrite (FeS2), and kaolinite (Al2Si2O5(OH)4).
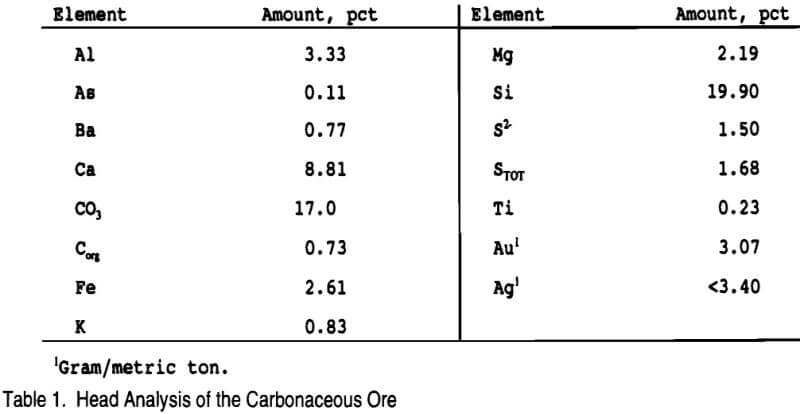
All bottle roll tests were conducted in 1 L large mouth plastic bottles with 50% solids (250 g of -100 mesh ore, 250 mL leach solution) and at ambient temperature. Deionized water and reagent grade chemicals were used in all tests. Thiosulfate was added as sodium thiosulfate (Na2S2O3·5H2O); copper as copper sulfate (CuSO4·5H2O); sulfite as sodium sulfite (NaSO3); and ammonia as ammonium hydroxide (NH4OH). In the cyanidation tests lime (CaO) was initially added to increase the pH above 10. After the pH was checked, sodium cyanide was added (NaCN) to the slurry. Oxygen was eliminated in some of the thiosulfate screening tests by purging the leach slurry with argon for 5 -10 min and sealing the bottle for the remainder of the test. The inert argon atmosphere was tested to provide an indication of what may occur within oxygen depleted zones of gold heap leaches. Once all the chemicals and ore had been added to the bottle, the slurry pH and Eh were measured and recorded. The bottle was then placed on the rolls for a specified amount of time.
At the conclusion of the test, the slurry was filtered using Whatman No. 5 paper and washed with 250 mL deionized water. The pH and Eh of the solution were again measured at the end of the leach after filtering, while the solids were dried at 90°C for 24 h, and weighed. Leach solutions were analyzed for precious metals using atomic absorption (AA) methods; copper by inductively coupled plasma (ICP); thiosulfate, sulfite, and sulfate by ion chromatography (IC); and ammonia by wet chemical methods. Leach residues were analyzed for precious metals using fire assay-ICP methods and sulfur species (SO4²-, S²-, S°, STot) using standard wet chemical methods.
Column tests were conducted using reagent grade chemicals and deionized water. Minus 6.4 mm ore was loaded in 14 cm inside diameter by 75 cm tall plexiglass columns for each test. The ore was sometimes agglomerated using 1.5 kg/t Portland cement. The target solution application rate was 3.12 mL/min, which is common within the gold industry. In most cases, fresh solution was applied to the top and allowed to flow down through the column where it was collected at the bottom. Continuously recycled solution was also used, as specified. Solution samples were taken daily and measured for pH, Eh, and sent in for a full analysis. Analytical methods were the same as those used for the bottle roll tests given above.
Once the leaching was complete, rinsing studies were initiated. These tests used tap water (pH 7 to 7.5) as the rinsing medium. The solution application rate was the same as during the leaching phase. Again, collected solutions were sent in for a complete analysis to determine the rinsing requirements for this ore. Tails from the column tests were subsequently analyzed for Au and Ag using fire assay-ICP methods and sulfur species using wet chemical methods.
Procedures and test conditions unique to a particular experiment are explained at the beginning of the appropriate section.
Results and Discussion
Cyanidation Tests
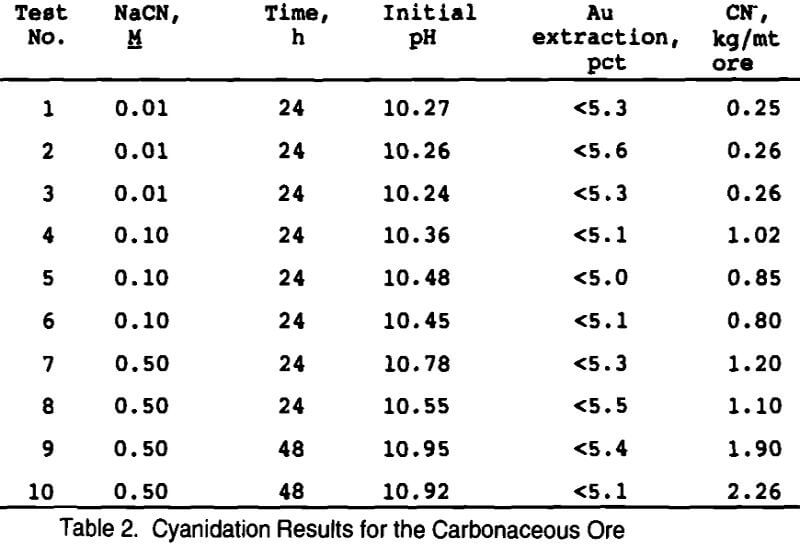
Standard cyanidation bottle roll tests were initially conducted to determine the baseline precious metals extraction and reagent consumption. This also gives an indication of the extent of the refractory nature of the low grade carbonaceous ore.
A total of ten bottle roll tests were conducted using cyanide concentrations ranging from 0.01 to 0.50 M and leaching for 24 or 48 hours. None of the cyanide tests were successful in extracting gold from the carbonaceous ore, as seen in Table 2. The first three tests were done in triplicate at 0.01 M cyanide, but more than 99 pct of the cyanide was consumed in the leach, indicating that stronger concentrations would be required. Tests 4-6 were also done in triplicate, but at a higher cyanide concentration of 0.10M. Cyanide consumption was only 30 pct for these tests, indicating that cyanide concentration was not the limiting factor for gold extraction. Increasing the cyanide concentration to 0.5M still did not extract any gold, but slightly higher cyanide consumptions were realized. As a last attempt, the final two tests were conducted at longer leach times (48 h) in order to be more comparable to the thiosulfate leaching tests. No gold was extracted, but cyanide consumption increased due to the longer leach time. This carbonaceous ore was found to be very refractory to direct cyanidation.
Two cyanidation tests were also conducted on roasted ore (at 500°C for 2½ hours in an air atmosphere) to determine the best possible extraction from this ore at -100 mesh. Results showed that 82 pct of the gold was recoverable while consuming 2.2 kg CN-/mt ore.
Screening Tests
Statistically designed experiments provide a large amount of information about a leaching system with a minimal number of tests. This experimental method is especially useful in the thiosulfate system, since there are numerous interactions among the various reagents (Byerly, et al., 1973, Tozawa, et al., 1981, Langhans, et al., 1992, Li, et al., 1995). A screening design was first used to determine the effects each of the variables had (independent from one another) on gold extraction and thiosulfate consumption, as well as to provide information on interactions. This design is used as a coarse adjustment of the leaching parameters. The next step was to eliminate some of the insignificant variables used in the screening tests and proceed with surface response experiments. This phase was a fine tuning of the leaching parameters and was used to determine the optimum thiosulfate leaching conditions. Standard bottle roll tests were used to generate the data in all of the statistical experiments.
A 2-level factorial design with 1/8 replicates of 7 factors (variables) was used for the screening experiments (this is also known as a 2 7 3 fractional factorial design). This design requires a total of twenty tests to be conducted, including four at the center point (medium range) in order to provide information about error associated with the experiments. Table 3 shows the specific variables used in the screening experiments along with their ranges. These ranges were chosen based on some initial tests conducted in the thiosulfate system, and a review of the literature.
Table 4 shows the results from the factorial screening design experiments. The table has been arranged in order of
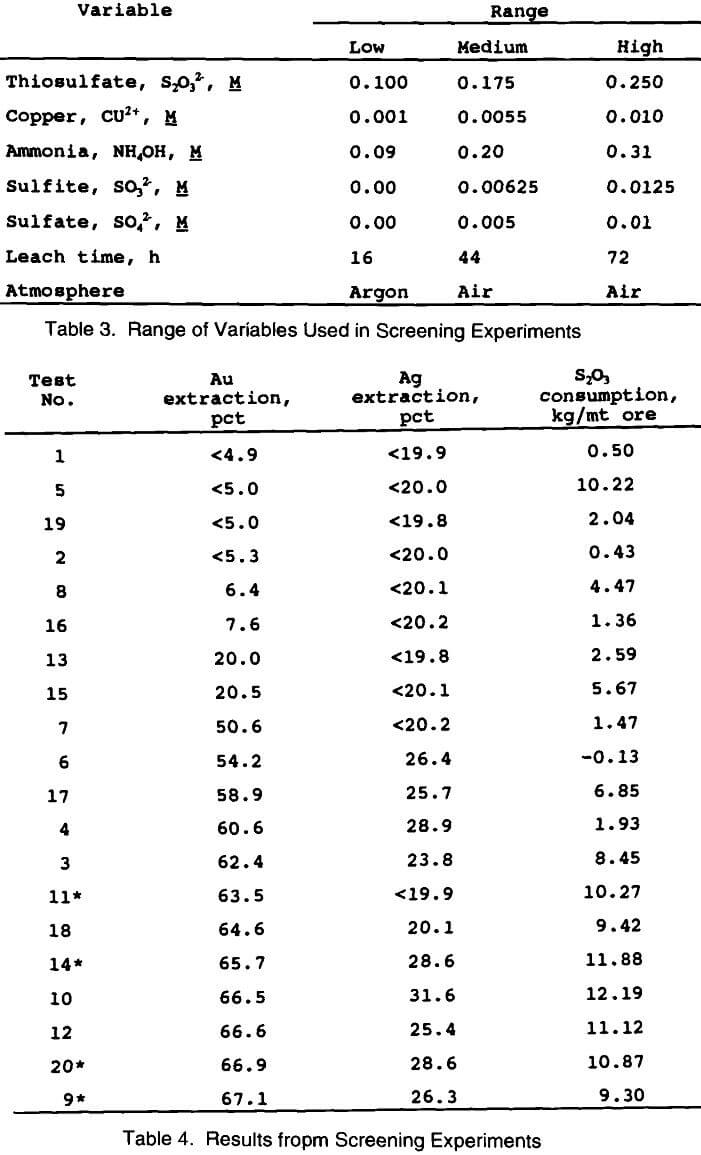
increasing gold extraction to allow for direct comparison with thiosulfate consumption. It is immediately apparent that under the proper conditions thiosulfate is a much better lixiviant for extracting gold from this carbonaceous ore than cyanide. Without complete optimization of the leaching variables, thiosulfate extracted about 65 pct of the gold, compared to zero for cyanide. It is also apparent that the thiosulfate consumption generally increased with gold extraction.
The four center points used in this experimental design provide an indication of the precision of the experimental and analytical procedures. The average gold extraction at the center of the design was 65.8 ± 2.7%. The precision of the reported gold results is therefore ± 2.7%. The average thiosulfate consumption at the center point was 10.58 ± 1.17 kg S2O3/mt ore. The precision of the reported thiosulfate results is ± 1.17 kg/mt ore.
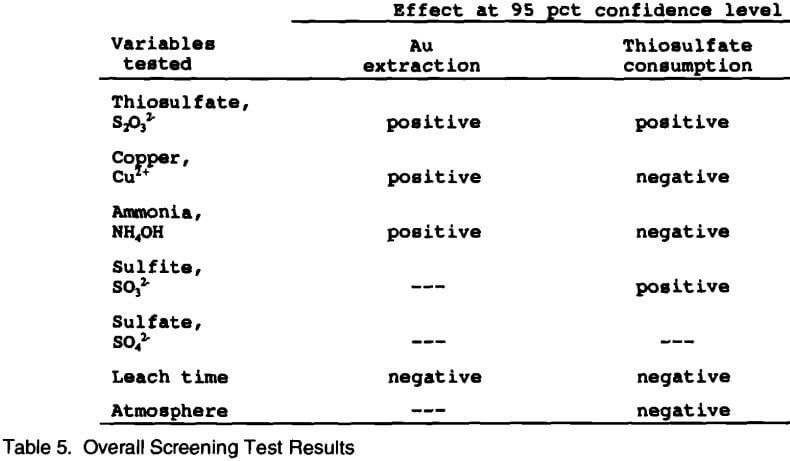
A summary of the analysis from the screening experiments is given in Table 5. All data from the screening experiments were analyzed at the 95 pct confidence level with respect to gold extraction and thiosulfate consumption. This means that there is only a 5 pct chance that the effect is due to error. Thiosulfate, copper, and ammonia all had a positive effect on gold extraction. In other words, at their high concentration, gold extractions increased. The leaching time had a slight negative effect in that gold extractions decreased with longer leach times. Sulfite, sulfate, and leaching atmosphere had no significant effect on gold extraction within the ranges tested. Copper, ammonia, leach time, and leach atmosphere all had a negative effect on thiosulfate consumption at their high levels. That is, lower concentrations (or levels) of these variables would minimize thiosulfate consumption. Both thiosulfate and sulfite concentrations had a slight positive effect on thiosulfate consumption (minimized consumption) at their high levels, while sulfate had no significant effect within the experimental range tested.
Optimising Leaching Tests
A significant amount of curvature was detected for both gold extraction and thiosulfate consumption in the screening tests, indicating that a response surface design would have to be run in order to define the role of significant two-factor interactions. This type of design requires a higher number of tests to be run per variable, so it was limited to four. From the information provided by the screening design, those four variables included thiosulfate, copper, and ammonia concentration, and leach time. The ranges used for these variables in the response surface tests were identical to those used in the screening design (Table 3). Sulfite was set at its medium value (0.00625 M) in order to minimize thiosulfate consumption, sulfate was kept at its low value (zero) to minimize reagent costs, and the leaching atmosphere was air in order to simplify the experiments. This (air) also more closely approximates actual heap leaching conditions.
Data from the response surface tests were analyzed at the 90 pet confidence level with respect to both gold extraction and thiosulfate consumption. Table 6 shows the analysis with respect to gold extraction. The probability for error gives information on whether the effect seen is significant or just due to experimental and associated error. For example, the probability for error for thiosulfate is 0.003 or a 0.3% chance that the effect of thiosulfate on gold extraction can be attributed to random error. A smaller probability for error number also corresponds to a greater effect on the response being measured. The table is arranged in order of decreasing effect on gold extraction. Ammonia concentration was therefore the single most significant variable effecting gold extraction, while the thiosulfate-thiosulfate interaction was the least significant.
The following reduced quadratic model equation (1) was generated from the data in Table 6 to describe gold extraction within the experimental region as a function of ammonia, copper, and thiosulfate concentrations, and leach time:
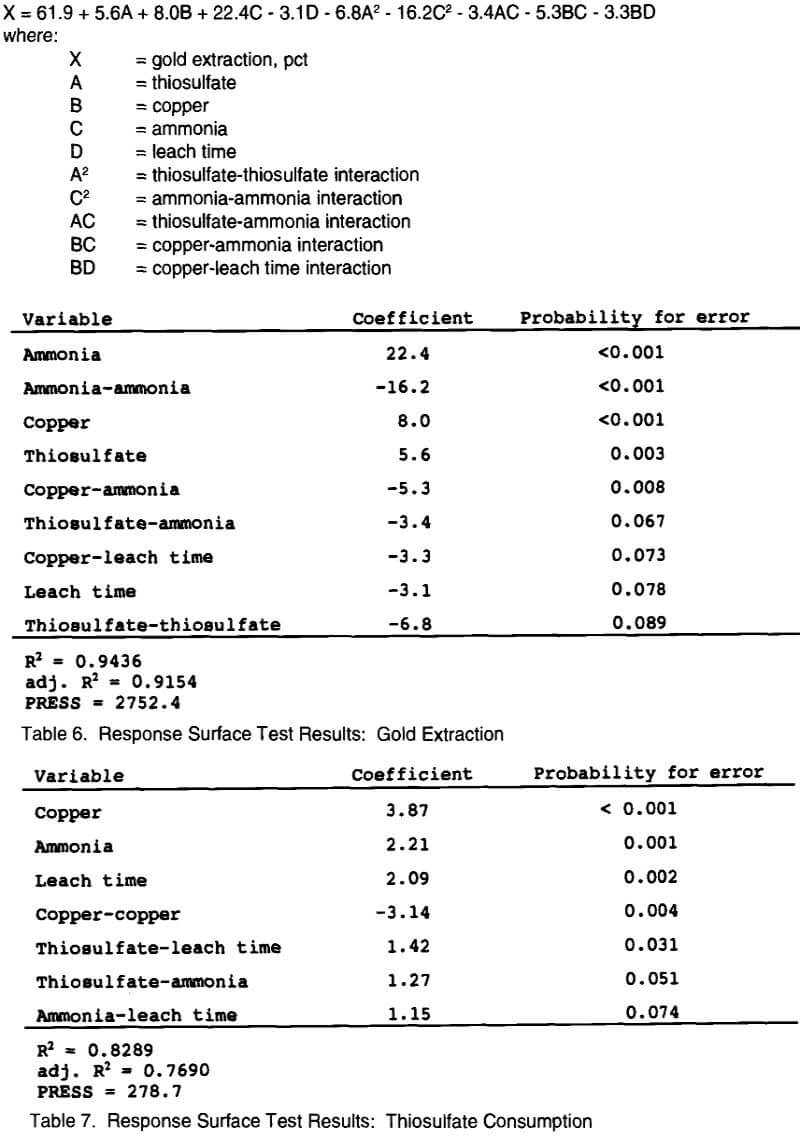
All variables have been normalized from -1 (low value) to +1 (high value) in this equation. This model shows a good fit to the actual data with a coefficient of determination (R² value) of 0.94 (the model accounts for 94 pct of the variance in the data). The adjusted R² value of 0.91 indicates that 91 pct of the variance is accounted for with this model over the entire experimental region. A PRESS (predicted residual sum of squares) statistic of 2752 indicates the amount of error associated with model predictions, a smaller number having less error. These statistical values indicate that the model given above has a very good fit to the data, but will have some error in predicted gold extractions generated from the equation.
An analysis of the data with respect to thiosulfate consumption is shown in Table 7. The table is again arranged in order of decreasing effect on thiosulfate consumption. Copper concentration had the greatest effect on thiosulfate consumption, as seen by its low probability for error value. The coefficient for copper is positive, which means that high copper concentrations increased thiosulfate consumption. This conclusion is consistent with what was observed in the screening tests. The ammonia-leach time interaction had the lowest impact on thiosulfate consumption, with increased concentrations leading to increased consumption. Although the screening tests showed that thiosulfate concentration had a positive effect on thiosulfate consumption, no significant effect was seen for this variable in the response surface tests. This suggests that the effect of thiosulfate concentration on thiosulfate consumption is minimal within the ranges tested.
The following reduced quadratic model equation (2) was generated from the data to describe thiosulfate consumption within the experimental region as a function of copper, ammonia, leach time, and thiosulfate:
Y= 10.15 + 3.87B + 2.21C + 2.09D – 3.14B² + 1.27AC + 1.42AD + 1.15CD…………………………..(2)
where:
Y = thiosulfate consumption, kg/mt
B = copper
C = ammonia
D = leaching time
B² = copper-copper interaction
AC = thiosulfate-ammonia interaction
AD = thiosulfate-leaching time interaction
CD = ammonia-leaching time interaction
All variables have been normalized from -1 (low value) to +1 (high value) in this equation. The coefficient of determination (R² value) for this model is 0.83 (the model accounts for 83 pct of the variance in the data). The adjusted R² value of 0.77 indicates that 77 pct of the variance is accounted for with this model over the entire experimental region. The PRESS statistic (278.7) is very low, indicating only a small amount of error is associated with the model predictions. Again, these statistical values indicate that the model given above has a good fit to the data and is a valid conclusion for analysis of the data with respect to thiosulfate consumption.
In order to find the optimum region for maximum gold extraction and minimum thiosulfate consumption, the contour plot generated from equation 1 was overlaid on the contour plot generated from equation 2. The resulting plot showing the optimum region (shaded) is given in Figure 1. The response surface experiments predict that at 0.25M thiosulfate,
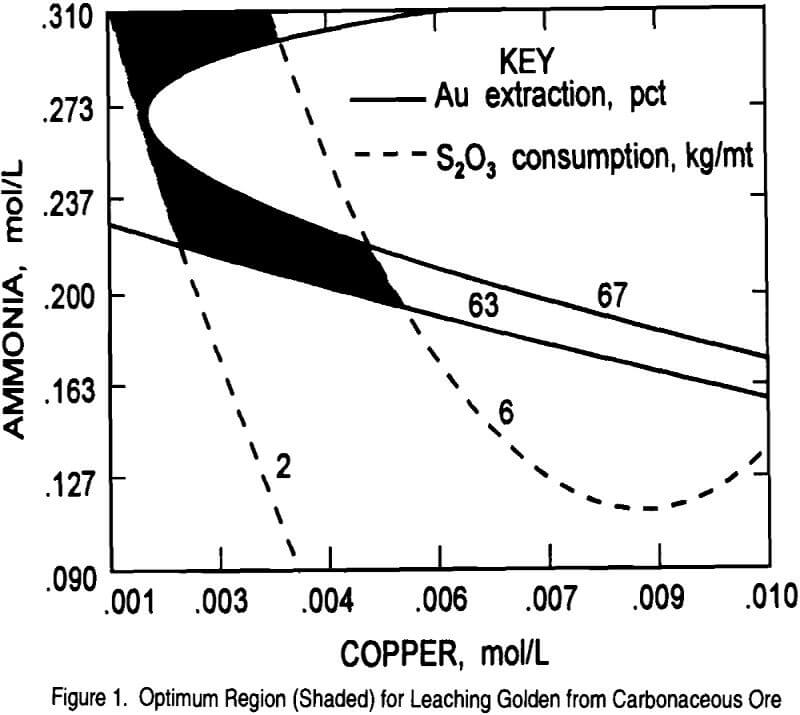
0.27M ammonia, 0.0014M copper, 0.00625M sulfite, and leaching for 16 hours a gold extraction of 67 pct with 2 kg thiosulfate/mt consumption would be obtained. Results from some of the response surface tests have already proven the feasibility of extracting 67 pct of the gold, but thiosulfate consumption was higher (5 – 24 kg/mt) than the predicted value of 2.
A series of three identical bottle roll tests were conducted at these specified optimum conditions for verification of the prediction. Results showed that 62 ± 1.6 pct of the gold could be extracted while consuming only 0.93 ± 0.76 kg thiosulfate/mt ore. This consumption is half that predicted in the model, while gold extraction decreased only slightly.
Column Tests
Research was scaled up from bottle roll tests to columns in order to both confirm and adjust the leach conditions required to achieve maximum gold extraction with minimum thiosulfate consumption at a larger particle size. These tests also provide information that can be applied to field scale operations. Rinsing tests were conducted in conjunction with the leaching tests in order to provide information on closure of thiosulfate leached heaps.
Table 8 gives the conditions used in the column tests. The first column tested the optimum conditions as defined in prior bottle roll tests (0.25M S2O3, 0.0014M Cu, 0.00625M SO3, and 0.27M NH4OH). The second test used similar conditions with the exception of decreasing the thiosulfate concentration to 0.15M. The third column was identical to the second, except that the ore was agglomerated with Portland cement. Column four also used agglomerated ore, but the ammonia concentration was decreased to 0.15 M compared to column three. Column five was a repeat of column one, with the exception of agglomerating the ore and increasing the sulfite concentration to 0.0125M. Column six was a duplicate of column three, except that the sulfite concentration was doubled to 0.0125M. This column also used continuously recycled leach solution to extract the precious metal values, which more closely simulates the conditions used in prior bottle roll tests.
Table 9 summarizes the results from the column leaching tests on the low grade carbonaceous ore. Increasing the particle size from minus 100 mesh (bottle roll tests) to minus 6.35 mm (column tests) did not result in decreased gold ex-
tractions. In fact, all of the columns yielded higher gold extractions (69 to 78 pct) compared to that found in the bottle roll tests (62 pct). This can be attributed to more complete leaching of gold from the carbonaceous material with each new application of thiosulfate and to longer leach times used in the column tests. Although the column leach times were longer than the bottle roll tests, they were still reasonable for this amount of ore, ranging from 6 to 10 days in columns using fresh leach solution. Column six, which reused the same leach solution continuously without replenishing any reagents, took 2.5 to 4 times longer to leach the gold. However, gold concentration in the leach solution was also significantly increased by using recycled solution, going from approximately 2.5 mg/L to 18.5 mg/L, which may be more desirable for an operation utilizing cementation for metal recovery (Li, et al., 1995).
Decreasing the thiosulfate (column 2) and ammonia (column 4) concentrations had little effect on gold extraction, indicating that commercial operations may be able to operate at lower chemical concentrations, which would result in a significant initial chemical cost savings. Agglomeration of the ore was also found to have an insignificant effect on gold extraction. This suggests that commercial operations with clay in their ores would still be able to agglomerate without adverse effects to the leaching system. In fact, none of the adjustments made to the leaching conditions had a large impact on gold extraction.
There was a lot more variability and impact on the reagent consumption results. Overall, consumption was higher in the column tests than in the bottle roll tests. Thiosulfate consumption ranged from 0.4 to 5.4 kg/mt ore in the columns compared to 0.93 kg/mt ore in the optimum bottle roll test. Most of the reagent consumption in the column tests occurred upon the initial application of reagent to the ore. For unagglomerated ore, 60 to 70 pct of the total consumption occurred on the first day, while the agglomerated ore consumed only 33 to 40 pct of the total. Agglomeration with cement has a tendency to increase the solution pH, possibly buffering the solution. This indicates that initial consumption may be reduced with addition of a buffer to the leach solution or preconditioning the ore to pH 10 using lime or caustic.
It is interesting to note that from 23 to 55 mg/L Cu in the leach solution was lost to the ore during the course of leaching. This copper catalyst will have to be replenished before the leach solution can be recycled. The ratio of copper lost to gold recovered in the leaching stage is also provided in Table 9. The cost of restoring copper to the leach solution will have to be accounted for in the economic analysis of this process.
Following the thiosulfate column leaching tests, rinsing studies were conducted on the barren ore to define conditions needed to meet Federal drinking water standards (EPA, 1986). Tap water (pH 7 to 7.5) was used as the rinsing media in all columns. Thiosulfate solutions must specifically meet copper (1.0 mg/L), sulfate (250 mg/L), and pH (6.5 to 8.5) requirements, as well as concentration limits for other metal ions such as As, Fe, Ag, and Zn prior to effluent discharge. No limits have yet been defined for thiosulfate and ammonia.
Table 10 summarizes the results from the rinsing studies. All of the columns took only 2 days and 470 to 860 L H2O/ mt ore to meet the standards for copper. This suggests that the copper lost in the leaching cycle is stable and will not present a problem upon closure of the operation. Sulfate limits were met at 2 to 4 days and 600 to 1370 L H2O/mt ore in columns 1 through 5. In column 6 where the leach solution was continuously recycled, the time frame and amount of rinse solution required to meet the standards for sulfate tripled compared to the other five columns. The reason for this increase in rinsing conditions is probably related to having a higher concentration of sulfate present in the recycled leach solution, and adsorption of the sulfate species into ore particles. In the first four columns the pH limits were met in 8 to 11 days and after 2500 to 3500 L H2O/mt ore had been applied. Columns 5 and 6 required only 4 days and 1700 to 1850 L H2O/ mt ore to meet the standards. This can be attributed to differences in the experimental procedure and characteristics of the thiosulfate system. The barren ore in columns 5 and 6 was allowed to sit for a month before the ore was rinsed. In this time frame, thiosulfate within the column had begun to degrade, producing sulfate, which, in turn decreased the pH of
the column. This suggests that a delay in rinsing after the leaching cycle may help to meet pH limits for closure. These results indicate that no extraordinary problems should arise during the closure phase due to the use of thiosulfate.
Conclusions
The feasibility of using thiosulfate to directly recover gold from refractory low-grade carbonaceous ores was proven. Statistical methods were used to define the effects of the variables on both gold extraction and thiosulfate consumption. Two models were developed to describe gold extraction and thiosulfate consumption as a function of the leaching parameters. At 0.25M S2O3, 0.27M NH3, 0.0014M Cu, 0.00625M SO3, and leaching for 16 h, the models predicted a gold extraction of 67 pct with a consumption of 2.0 kg S2O3/mt ore. Actual tests conducted at these conditions resulted in 62 pct gold extraction with 0.93 kg thiosulfate consumed/mt ore. This compares to no gold extraction using a direct cyanide leach and 82 pct using cyanide on roasted ore. Column leaching tests with thiosulfate further demonstrated that 69 to 78 pct of the gold could be directly recovered while consuming 0.4 to 5.4 kg thiosulfate/mt ore. Continuous recycle of the leach solution led to increased leaching times for comparable precious metal recoveries, but also significantly increased the concentration of gold in solution. Results from rinsing studies suggest that closure of thiosulfate leached heaps should be straightforward.
