Table of Contents
An investigation of a particle agglomeration technique as a means of increasing the percolation rate of leaching solutions through heaps of clayey or finely crushed, low-grade, gold and silver ores. Bench-scale and pilot-scale experiments conducted on different ores indicated that the percolation rate of cyanide leaching solution was enhanced markedly by mixing the ore with a binder, such as lime or Portland cement, moistening the mixture, then mechanically agglomerating and aging the feed prior to heap building and leaching. In addition, the rate of silver and gold extraction markedly increased without sacrificing total recovery of values. The use of concentrated cyanide solution instead of water during the agglomeration procedure substantially decreased the leaching time required to obtain maximum recovery. Results from bench-scale and pilot-scale experiments using the particle agglomeration technique are discussed.
Exploration during the past few years has identified numerous low-grade, gold-silver deposits throughout the Western United States. Recent increases in gold and silver prices have generated a great deal of interest in processing these low-grade ores by heap leaching. Heap leaching with cyanide has potential application to many of these low-grade materials; however, if heap leaching is to be successful, the material must maintain good permeability after being stacked into heaps, so that the cyanide leaching solution percolates evenly throughout the heaps.
In an earlier publication, the Bureau of Mines described the advantages of particle agglomeration as a pretreatment for certain materials which are difficult to treat by standard heap leaching. Excessive amounts of clay or fines generated during crushing prevented uniform percolation flow of the leaching solution. Laboratory tests showed that the percolation flow rates of cyanide leaching solutions were enhanced markedly by mixing the ore with controlled amounts of lime and water, agglomerating, and then aging the mixture prior to the heap leaching step.
The objective of the present investigation was to investigate Portland cement (type II) as a binder in the particle agglomeration pretreatment and to transfer the technology to industry by cooperative pilot-scale studies in actual heap leaching operations.
Laboratory Equipment and Material
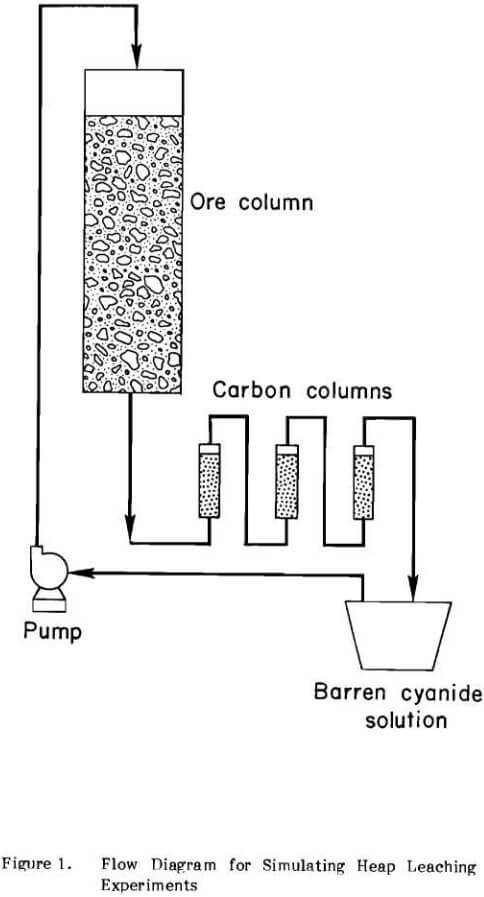
Investigations to determine the effectiveness of portland cement as a binder for the agglomeration of fine particles were carried out in column leaching experiments on 50-lb charges of material. A schematic of the laboratory apparatus is shown in Figure 1. The plexiglass columns were 5 ft high and had an inside diameter of 5.5 in. A removable porous support, 4 in. thick, was prepared from washed sand and gravel, which was enclosed in a 35-mesh nylon screen, and was placed at the bottom of the leaching column to prevent the ore from plugging the solution outlet. A 50-lb charge of material was placed on top of the porous support and gave a bed height of about 4 ft. A three-way plastic discharge valve was used to measure the flow rate and take solution samples. The pregnant cyanide solutions were pumped through three 1.5-in. diameter columns in series, each of which contained 30 grams of 6×16 mesh coconut shell activated carbon which adsorbed the gold and silver. The barren solution was recycled to the top of the column of ore.
The clayey gold ore described in RI 8388 was also used in this laboratory investigation. A 39-in. rotating disk pelletizer was used to agglomerate the feed for the columns. Binder, dry ore, and a controlled amount of water or strong cyanide solution were mixed and agglomerated. The agglomerated material was aged prior to leaching. After curing, the feed was placed in the column and leaching started by pumping solution onto the top of the column.
Investigation of Process Variables
Both lime and portland cement can be employed as binders for agglomerating fine and clayey particles.
Portland cement additions of 5 to 10 lb per ton of clayey ore produced exceptionally stable agglomerates with high porosity. Binding properties of the portland cement were superior to those exhibited by lime. The agglomerates produced using cement as a binder endured percolation leaching using spray or flooding methods to simulate heap and vat leaching, respectively. No fines migration or solution channeling were observed. Therefore, research investigations concentrated on portland cement as the binder.
Three principal process variables were investigated and were 1) the amount of binder mixed with the dry feed, 2) the curing time required for the hydration of the calcium silicate bridges, and 3) the amount of moisture used to wet the dry mixture.
Effect of the Amount of Portland Cement added on Percolation Flow Rate
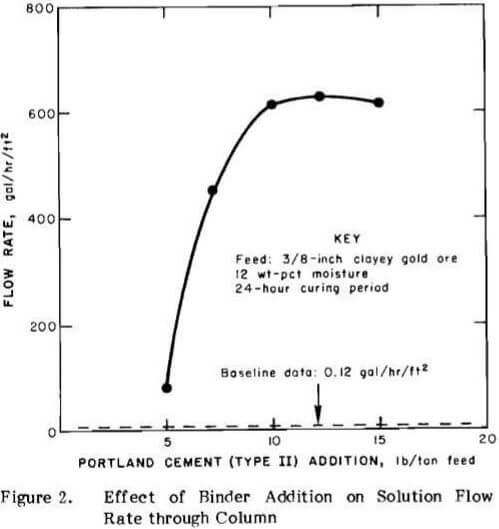
Baseline Data. Initial column heap leaching tests were conducted on the clayey gold ore to obtain baseline data for comparison with data obtained from agglomerated feeds. Different amounts of cement were added to 50-lb charges of clayey gold ore crushed to a nominal 3/8-in. feed size, but the material was not agglomerated. The cement provided protective alkalinity and showed the effect of Portland cement addition without agglomeration. The amount of cement was varied from 0 to 20 lb/ton of feed. After mixing the cement with the dry feed, the mixture was placed in the leaching column, and the downward percolation leach was started using 12 liters of solution containing 2 lb of sodium cyanide per ton. The leaching solution was recirculated through the system until no additional gold was detected in the pregnant solution. Flow rate measurements were made daily for five days and were averaged to determine the flow rate. The results showed that the percolation flow rate was independent of the amount of portland cement added to the dry feed. The flow rate remained constant at 0.12 gal/hr/ft² at cement additions from 0 to 20 lb/ton of feed. The results are shown graphically as baseline data in Figure 2.
Effect of Portland Cement Addition on Agglomeration
Fifty-pound charges of the clayey gold ore crushed to a nominal 3/8-in. size were mixed with from 0 to 15 lb of cement per ton of feed. Water was added to the cement-ore mixture until the moisture content of the mixture on the 39-in. rotating disk pelletizer was 12 wt.%. The wetted mixture was tumbled on the pelletizer until the fine and/or clay material was agglomerated. Adequate mixing of the cement and water into the ore was important for successful agglomeration of the feed. During the agglomeration step, the fine particles adhered to the surface of the larger particles; thus, avoiding particle segregation. The coarse particles were coated by fine particles. Coating of the coarse particles did not affect precious metal recovery. The fines coating the larger particles were porous enough so that cyanide solution could penetrate and dissolve the micron size gold particles associated with the coarser material.
The agglomerated feed was placed in the leaching column and cured for 24 hours at ambient temperature. The column was capped to minimize drying of the pellets. An increase in agglomerate strength was observed if the cement-ore mixture remained moist during curing. If the pellets dried during curing, the hydrolysis reaction stopped and partial breakdown of the agglomerates occurred upon wetting.
After curing, downward percolation leaching of the agglomerated material was initiated as described above. During cyanidation, no additional base was required to maintain the leach solution pH at 11. Figure 2 shows that increasing the amount of cement up to 10 lb/ton of feed markedly improved percolation rates through the column of ore. Figure 2 also indicates that high flow rates are achieved by agglomerating the clayey gold ore with 7.5 to 10 lb of portland cement per ton of ore. This amount of cement will supply the protective alkalinity required during leaching under normal heap conditions. Maximum flow rate measurements were made after leaching was completed. The material in the column was flooded with leaching solution, and the rate at which the solution drained from the column was measured.
The high percolation flow rates obtained under flooded conditions would be impractical for solution pumping requirements in an actual heap leaching operation, but the data demonstrate that very stable, porous agglomerates are produced and do not break down under exaggerated leaching conditions. The data also indicate that vat leaching techniques could be employed for leaching when portland cement is used as a binder.
Effect of Curing Time on Percolation Flow Rate
A series of column leaching experiments was conducted to determine the effect of curing time on the percolation flow rates. Fifty-pound charges of clayey gold ore were mixed with 10 lb of cement per ton of ore, wetted with 12 wt.% of water, and agglomerated on the disk pelletizer. The agglomerated charges were cured for 0, 2, 4, 8, 16, 24, and 36 hours at ambient temperature and in capped leach columns. Flow rates are presented in Figure 3.
The data show that the duration of the curing process is an important operating parameter. Percolation rates increased markedly up to eight hours of curing, but no further improvement occurred after eight hours. Curing the agglomerated feed for eight hours resulted in a flow rate of 600 gal/hr/ft² compared to the baseline flow rate of 0.1 gal/hr/ft², or a 6,000-fold increase in percolation. When lime was used as a binder, the best curing time was 24 hours.
Effect of Water Addition on Percolation Flow Rate
These experiments were performed on 50-lb charges mixed with 10 lb of cement per ton of feed. A 24-hr curing
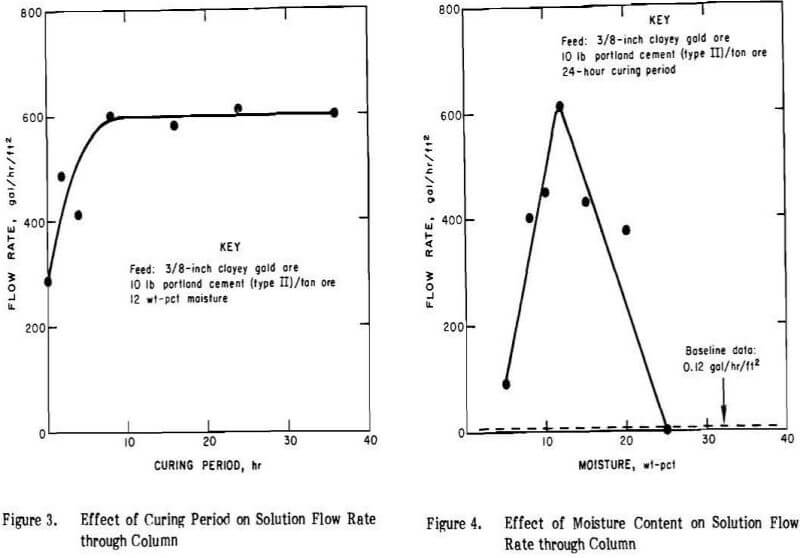
period was used as an experimental convenience even though only eight hours were needed to achieve full curing. The results are shown in Figure 4. Solution flow rate through the column of agglomerated ore increased considerably with increasing moisture content, attained a maximum of about 600 gal/hr/ft² at 12 wt.% moisture, and then rapidly decreased to less than baseline at 25 wt.% moisture. These data indicate that the permeability of the agglomerated feed is greatly dependent upon the amount of water employed in the agglomeration step. If too much water is added, the feed-cement mixture becomes a mass of mud, and does not produce stable, porous agglomerates.
Summary
The best combination of cement, water, and curing time determined experimentally for the clayey gold ore is summarized in Table 1. Agglomeration pretreatment

increased the percolation rate from 0.1 to 600 gal/hr/ft² of cross sectional area. Gold extraction was determined during the simulated heap leaching sequence. The 70% extraction obtained was almost identical to that obtained by agitation bottle leaching tests of 3/8-in. feed material.
Effect of Wetting with Cyanide Solution on Leaching Period
The effect of wetting the cement-ore mixture with concentrated cyanide solution instead of water during the agglomeration step was studied. By adding the required sodium cyanide to the feed during agglomeration, the leaching period required for maximum precious metal extraction might be decreased because the cyanide would be reacting with the gold during the curing period. Subsequent percolation leaching with water would recover the gold values.
A series of three column leaching experiments using 50-lb charges of the minus 3/8-in. clayey gold ore was conducted. Baseline-unagglomerated leaching, particle agglomeration with cement and water, and particle agglomeration with cement and cyanide solution were evaluated. The baseline test was conducted by placing the dry ore into the column and leaching with 2 lb of NaCN per ton of solution or 1 lb of NaCN per ton of ore. Sodium hydroxide was used for protective alkalinity. The cement-water agglomeration experiment was performed by mixing 10 lb of portland cement per ton of dry ore and wetting with water to bring the final moisture content of the feed to 12 wt.%, agglomerating the mixture on a rotating disk pelletizer, and aging the agglomerated material for 24 hours prior to leaching with 2 lb of NaCN per ton of solution. The cement-cyanide agglomerates were prepared in the same manner as described above except that the feed was wetted with a cyanide solution containing 8.6 lb of NaCN per ton of solution, and leaching was started with water. Results from these experiments are shown in Table 2.
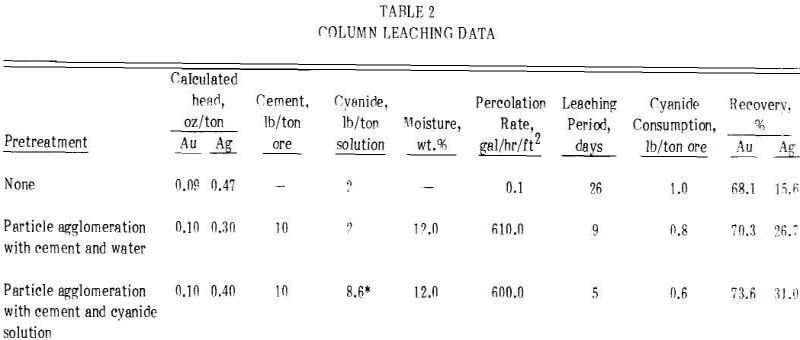
The data from these experiments show that agglomerating the ore with cement and strong cyanide solution decreased the leaching time for maximum gold extraction from 26 days for conventional heap leaching to five days for agglomeration heap leaching. Agglomerating the ore with cyanide solution decreased cyanide consumption.
Pilot Scale Testing of Agglomeration Technology
Several pilot scale tests employing particle agglomeration technology were conducted by private operators interested in applying the technique to their precious metal bearing material. Bureau of Mines personnel acted as advisors. Three pilot scale experiments will be described in this paper and were selected because of the diversity of the materials and methods of mechanical agglomeration employed. Feed materials used in the pilot scale agglomeration heap leaching tests included: 1) a gold ore containing few fines and no clays, 2) a clayey silver mine waste material, and 3) a silver-bearing mill tailings.
Gold Ore
An oxidized disseminated gold ore from an open pit mine located near Ely, Nevada, was employed in the first pilot scale experiment. The ore contained no clays and only 15 wt.% minus 20 mesh material when crushed to 80% minus 3/8 in. Although the ore did not require agglomeration pre-treatment to ensure adequate percolation rates, the operators were interested in the effectiveness of particle agglomeration for controlling particle segregation during stacking and leaching of the heap. They were also interested in decreasing channeling of solution during heap leaching.
Two pilot scale tests were performed. The first test was made on 5,000 tons of feed material crushed to 80% minus 3/8 in. and agglomerated with water to control the fines while the heap was being stacked.
The feed was prepared by wetting the dry ore with water at the discharge end of the crusher. Partial agglomeration of fines occurred by mixing with the coarser ore as it rolled down the sides of the stockpile. Additional agglomeration of fines occurred as the wetted feed was pushed over the edge of the heap and rolled down the heap’s sides. The feed material was carefully stacked on the heap in a manner described by Chamberlin.
Leaching was accomplished by sprinkling alkaline cyanide solution containing 0.5 lb of NaCN per ton over the heap surface. The application rate of cyanide solution was 0.18 gal/hr/ft² of heap area. The leaching solution percolated through the heap and was collected on a water-impervious pad which drained into a plastic-lined pregnant solution pond. The pregnant solution from the pond was pumped through a series of columns containing coconut shell activated carbon which adsorbed the gold values. The barren solution was pumped to a plastic-lined pond for recycle to the heap. Makeup reagents were added in the barren solution pond. Pertinent leaching data obtained are shown in Table 3.
The second test was conducted on 2,500 tons of ore crushed to 80% minus 3/8 in. The ore was mixed with 10 lb of portland cement per ton of dry ore, and wetted with a solution containing all the NaCN required for the leach. The agglomerated material was aged on the heap during heap construction.
The feed material was transported with a front-end loader from the crushed ore stockpile to a 9-ton weighing hopper. Cement was added to the ore as it was conveyed from the hopper to a conventional 14-yard-capacity concrete mixer. Cyanide solution was introduced into the mixer in three increments. The final moisture content of the 9-ton batches of agglomerated ore was between 7 and 10 wt.%. Agglomeration of the ore occurred during the transport of the treated ore to a stockpile area. The agglomerated feed was transferred from the stockpile by a front-end loader and dumped over the edge of the heap. The same leaching/carbon adsorption procedure employed in the first test heap was used except that no makeup reagents were required. Pertinent leaching data comparing both test heaps are shown in Table 3.
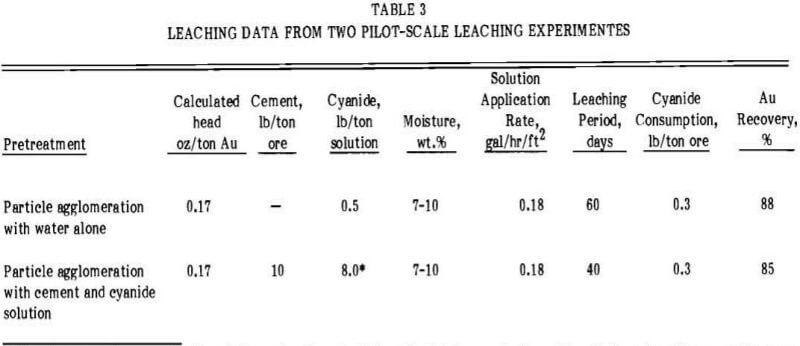
required to obtain the best gold recovery. Gold recoveries were the same as those obtained in small scale column percolation experiments. Particle agglomeration with water or with cement and cyanide solution was effective in controlling the migration of fines during stacking and leaching of the heap and in decreasing solution channeling during leaching.
Silver Mine Waste Material
The silver mine waste from the old Packard mine workings near Lovelock, Nevada, contained considerable fine material and required particle agglomeration in order to obtain an adequate flow rate through the stacked heap. Before applying particle agglomeration, a 30,00-ton heap of mine run waste was constructed on a water-impervious pad. When alkaline cyanide solution was applied to the heap, it ran off the sides of the heap rather than percolating through the bed of material. No silver was recovered from the material using conventional heap leaching. Several laboratory scale column leaching experiments were performed by the Bureau of Mines to evaluate the feasibility of applying particle agglomeration techniques to the material. Since results from the laboratory experiments were encouraging, the operators of the property conducted pilot scale particle agglomeration/heap leaching tests. Three 300-ton heaps were constructed and leached to determine the best feed size and leaching conditions. These tests showed that the feed had to be crushed to 80% minus 9/16 in. to liberate the silver values. Leaching with dilute cyanide solution for six days at a flow rate of 9 gal/hr/ft² of heap area achieved a 65% silver recovery.
Using the agglomeration and leaching conditions determined by the above tests, the operators constructed and leached a 1,000-ton heap. Dry material from the original 30,000-ton heap was transported to a hopper where it was conveyed to a two-stage roll crusher. The nominal 9/16-in. feed from the crusher was conveyed to the agglomerator.
Agglomeration was accomplished by wetting the crushed feed with a cement slurry. Ten pounds of cement per ton of feed were added, and the final moisture content of the agglomerated feed was maintained between 9 and 11 wt.%. The moistened feed tumbled down a vibrating inclined chute, which was 4 ft wide and 8 ft long and contained several “stair steps” to effect agglomeration.
The operators experienced difficulty in applying the cement in a slurry. Insufficient agitation in the cement-water mixing tank caused the cement to settle and the feed was wetted with only one quarter of the cement required for adequate agglomeration.
Another problem encountered in adding the cement as a slurry was the effective life of the wet cement. The cement hydrolyzes rapidly so that it is ineffective as a binder in less than five hours. However, even with the problems experienced by adding the cement as a slurry, the pilot-scale experiment was successful. The agglomerated feed was conveyed to a stockpile and later transferred with a front-end loader to a water-impervious leaching pad.
The 1,000-ton agglomerated heap was leached by sprinkling dilute cyanide solution over the heap at a rate of 9 gal/hr/ft² of heap area. The pregnant solution was collected on the water-impervious pad and drained into a plastic-lined pond. A Merrill-Crowe zinc precipitation method was used to recover the silver from the pregnant solution. The resultant barren solution was recycled to the heap. Sixty percent of the silver in the ore was recovered in six days of leaching.
Particle agglomeration with cement and water was a viable processing technique for this material, whereas conventional heap leaching was unsuccessful. The operators plan to incorporate this technology into their commercial operation. However, dry cement will be mixed into the feed, and the mixture moistened with a solution containing all the NaCN required to dissolve the contained silver.
Silver Mill Tailing
The third pilot scale test was performed on a silver mill tailing containing 2.5 oz of silver per ton. The tailings were from an old cyanide mill located near Virginia City, Nevada. The tailings which were 65% minus 200 mesh had been exposed to weathering for several decades.
Laboratory-scale column leaching experiments indicated that the material could not be leached using conventional heap leaching methods because the material was too fine. The tailings were too low grade to warrant construction of a conventional agitation cyanide plant. Several laboratory-scale agglomeration experiments were made and gave encouraging results. The operators decided to conduct a small pilot scale test to determine the effectiveness of agglomeration technology for recovering the contained silver values. Forty tons of silver-bearing tailings were mixed with 30 lb of portland cement per ton of feed, wetted with a strong cyanide solution so that the final moisture content of the feed was 22 wt.%, agglomerated in a rotating drum and then aged on the heap for several days prior to percolation leaching.
The feed was placed into a hopper and screw-fed to a rotating drum. The drum was 42 in. in diameter and 7 ft long. Dry cement was mixed with the tailings at the discharge end of the screw feeder. The mixture was moistened with cyanide solution applied through three spray nozzles mounted inside the balling drum. A scraper was used inside the drum to prevent material buildup on the walls of the drum. The agglomerates from the drum were conveyed to the heap.
Leaching was initiated by spraying water over the surface of the heap. The pregnant solution was collected on a water-impervious pad and drained into a pregnant solution reservoir. The pregnant solution was pumped upward through a series of five columns containing coconut shell activated carbon which adsorbed the silver values. The resultant barren solution was recycled to the heap. A silver recovery of 78% was obtained in five days of leaching at a percolation rate of 12 gal/hr/ft² of heap area. A silver recovery of 81% was obtained in the laboratory agglomeration experiments.
Particle agglomeration with cement and cyanide solution again demonstrated its effectiveness as a processing technique for materials that possess poor percolation characteristics. The quantity of cement used in the pilot scale test was more than required because laboratory results showed that 15 lb of cement per ton of feed was sufficient for adequate agglomeration. The operators of this property intend to use particle agglomeration/heap leaching to process the silver-bearing mill tailings.
Potential Benefits Using Agglomeration Techniques
Several potential benefits can be attributed to particle agglomeration pretreatment of ores and tailings before heap leaching:
- Clayey and finely crushed ores can be treated by low-cost heap leaching procedures. This permits treatment of large low-grade ore bodies, many of which are presently considered submarginal for cyanide mill construction.
- Increased gold recovery from an ore can be obtained because additional gold may be liberated by finer crushing without encountering particle segregation during preparation of ore heaps. Particle segregation in unagglomerated materials can cause localized accumulation of fines that inhibit flow of leaching solutions.
- Percolation rates are increased, thus decreasing the required leaching cycle. This means that the mine capacity can be increased without increasing capital cost for pad preparation.
- The highly porous nature of the agglomerates permits the heaps to “breathe,” thereby providing the oxygen necessary for the gold dissolution. The height of the heap can be increased so that the pad preparation cost per ton of ore processed is less, and land area is more effectively utilized.
- Favorable environmental conditions are realized in that the highly porous structure of the heap permits efficient washing of residual cyanide from processing heaps. The stable agglomerates minimize dusting problems when the heaps are abandoned.
- Precious metal concentration in solutions exiting from the heaps are greater. Efficiency of gold-silver recovery from these solutions by precipitation or adsorption on activated carbon is enhanced.
Conclusions
Solution flow rates through ore heaps are increased by agglomerating the fine particles in the ore with portland cement and water or cyanide solution, then curing. The agglomeration procedure eliminates the problem of particle segregation during the stacking of crushed ore and results in more rapid recovery of the precious metal values, smaller soluble gold and silver losses, and more thorough washing of the residual free cyanide in leached heaps. By improving percolation leaching, this technique has the potential of helping to solve environmental problems associated with tailings disposal and of making an important economic impact on gold-silver recovery operations.
What to do if your leach pad has Poor Percolation Characteristics?
https://www.911metallurgist.com/improving-percolation-rates-heap-leaching/
