Table of Contents
To insure the continuing viability of the domestic minerals and materials economy and the maintenance of an adequate minerals base, the U.S. Department of the Interior, Bureau of Mines, has been investigating the development of methods for improving gold and silver recovery from low-grade resources. Discoveries during the past decade of numerous low-grade, gold-silver deposits in the Western United States has stimulated development of low-cost cyanidation procedures for precious metal extraction from these resources. Heap leach cyanidation techniques possess considerable potential for application to these low-grade ores, small ore bodies, and mine strip waste material where fine grinding is not necessary for good extractions. For heap leaching to be successful, these resources must have good permeability, after being crushed and stacked into heaps, to achieve uniform distribution of the cyanide leach solution throughout the heaps.
Some of the most difficult of the gold and silver ores to treat successfully by heap leaching are those containing excessive amounts of clays, or fines generated during crushing. Presence of excessive amounts of slimes (generally classified as minus 50-micron or 270 Tyler mesh sieve size particles) in the heap leach feed will slow the percolation flow of the leach solution, cause channeling, or produce dormant or unleached areas within the heap. This may result in unreasonably long leaching periods and poor extractions. In extreme cases, the clays or slimes can completely seal the ore heap, causing the leach solution to run off the sides of the heap rather than to penetrate the ore heap.
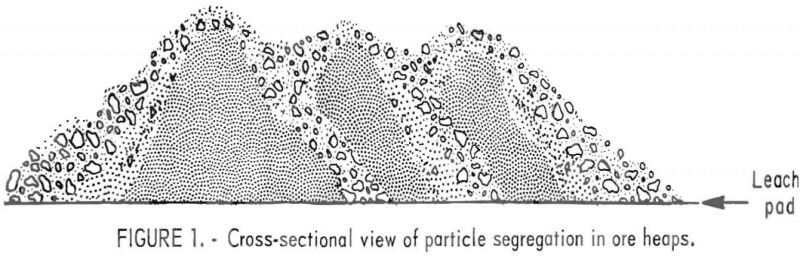
The problem of heap leaching ores with fines can be aggravated during stacking and preparation of the ore heaps because of the natural sorting of coarse and fine material that occurs during these steps. This phenomenon results in concentration of ore fines in the center of individual ore piles and concentration of the larger rock fragments on the lower slopes and base of the pile. Figure 1 illustrates this particle segregation effect. When the individual piles within the heap are leveled off for the installation of the sprinkling system that delivers the leach solution, additional segregation occurs as the fines sift through the coarser ore particles. The segregation results in localized areas or zones with marked differences in permeability, and as a consequence, the leach solutions follow the course of least resistance, percolating downward through the coarse ore regions and bypassing or barely wetting areas that contain large amounts of fines or slimes. Effective utilization of marginal gold and silver resources by heap leaching will require development of new methods to achieve more uniform size distribution during the preparation of the ore heaps and better slime control during leaching.
Agglomeration and balling of crushed ore to produce a porous and more uniform feed material for heap leaching is a potentially viable method for treating clayey ores. Little attention has been given to such methods for
improving uniform percolation flow of leach solutions through silver and gold ores. In 1905, T. C. Scrutton developed a unique technique for obtaining rapid vat leaching of a clayey ore in which the gold was finely disseminated. His technique consisted of rolling the ore down a chute inclined at 60 degrees to form agglomerates or balls readily permeable by the cyanide solution. However, these agglomerates lacked rigidity, and, to insure good percolation leaching and washing, they could not be bedded in layers more than 3 feet deep. If this depth was exceeded, difficulties were experienced in obtaining uniform leaching and washing, resulting in reduced gold recovery. Shepard and others in 1937 studied the addition of lime and carbon dioxide or calcium carbonate to gold-bearing tailings to form agglomerates suitable for vat leaching. Satisfactory percolation flow rates were achieved in 90-gram scale experiments, but the reagent requirements were prohibitive.
Agglomeration and pelletizing has been used in other segments of the mineral industry. This technique was first used successfully around 1911 for pelletizing iron ores. Since that time agglomeration and pelletizing (or briquetting) has been widely adopted for consolidation of fines for many other materials, such as manganese, fluorspar, and phosphates. These materials were pelletized to consolidate fine particles into large, dense, and mechanically strong masses, principally to prevent dusting problems during furnacing operations.
The objective of the present investigation was to evaluate particle agglomeration procedures for improving solution flow rates in the heap leach cyanidation process. The concept was to investigate procedures whereby loose-knit, polymerlike agglomerates could be formed that were highly permeable to solution flow and yet mechanically stable during the leaching sequence.
Equipment, Materials & Apparatus
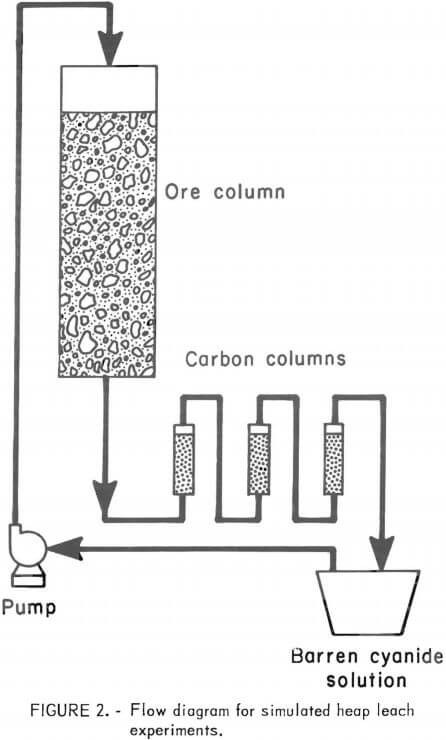 Initial investigations to determine the effectiveness of agglomeration techniques for enhancing the percolation characteristics of various types of problem ores were carried out in column leach experiments on 50-pound charges of feed material. A schematic of the laboratory apparatus is shown in figure 2. The Plexiglas columns were 5 feet high with an inside diameter of 5.5 inches. A removable diaphragm or porous support, 4 inches thick, was prepared from washed gravel and sand, and enclosed in a 35-mesh nylon screen. It was placed on the bottom of the leach columns to prevent the ore from plugging the outlet. A 50-pound charge of test material was placed on top of the porous support to make a bed about 4 feet in height. A three-way plastic discharge valve was employed to facilitate measurement of the flow rate, and to enable taking solution samples. The pregnant cyanide solutions were pumped through three 1.5-inch-diameter columns in series, each of which contained 30 grams of 6 x 16 mesh activated carbon, manufactured from coconut shells, to recover the gold and silver values by adsorption. The barren solution was recycled to the top of the column of ore.
Initial investigations to determine the effectiveness of agglomeration techniques for enhancing the percolation characteristics of various types of problem ores were carried out in column leach experiments on 50-pound charges of feed material. A schematic of the laboratory apparatus is shown in figure 2. The Plexiglas columns were 5 feet high with an inside diameter of 5.5 inches. A removable diaphragm or porous support, 4 inches thick, was prepared from washed gravel and sand, and enclosed in a 35-mesh nylon screen. It was placed on the bottom of the leach columns to prevent the ore from plugging the outlet. A 50-pound charge of test material was placed on top of the porous support to make a bed about 4 feet in height. A three-way plastic discharge valve was employed to facilitate measurement of the flow rate, and to enable taking solution samples. The pregnant cyanide solutions were pumped through three 1.5-inch-diameter columns in series, each of which contained 30 grams of 6 x 16 mesh activated carbon, manufactured from coconut shells, to recover the gold and silver values by adsorption. The barren solution was recycled to the top of the column of ore.
A process development unit (PDU) used to further quantify results obtained on a bench scale contained the same arrangement of equipment as shown in figure figure 1. The column was 24 inches in diameter and 16 feet high. Tests were conducted on 1.5 tons of ore, which filled the column to a height of 10 feet. Each carbon adsorption column held 5 pounds of 6 x 16 mesh activated carbon. A silver mill tailing and a clayey gold ore with poor percolation characteristics were employed in the preliminary studies. This gold ore was also studied in the larger scale PDU percolation experiments.
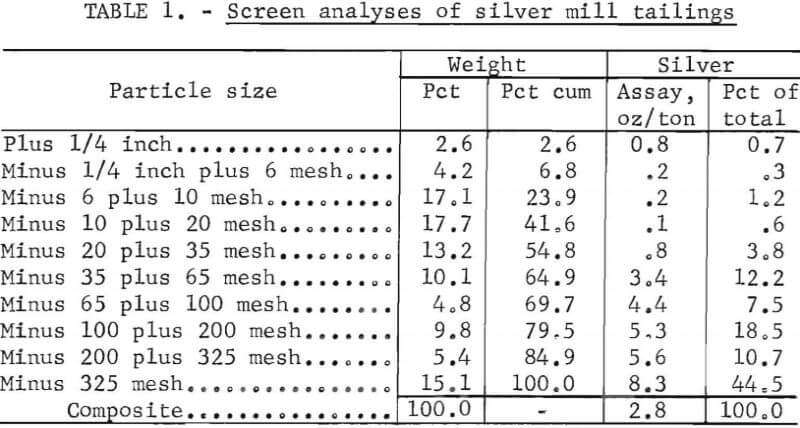
The silver mill tailing was a siliceous material containing quartz, sodium-potassium feldspars, biotite, and small amounts of clays and surface carbonates. The tailings material contained 2.8 ounces of silver per ton. Table 1 shows the particle size and silver distribution. Approximately 30 wt-pct of the feed was minus 100-mesh fines, that carried three- fourths of the total silver values.
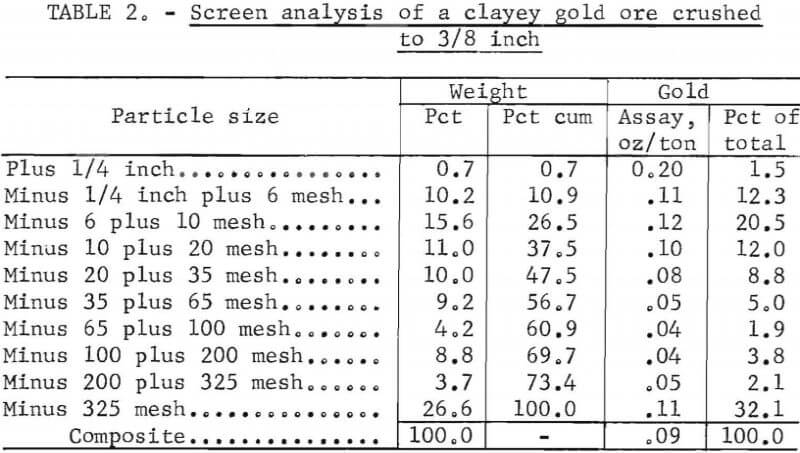
The gold ore contained a large amount of clay minerals and had very poor percolation characteristics The ore was an altered igneous rock containing quartz, feldspars, altered biotite, epidote, clays, and earthy iron oxides. It carried 0.09 ounce of gold per ton. The leachable gold portion was readily soluble in dilute cyanide solution. Screen analyses data on the ore crushed to a nominal 3/8-inch feed are shown in table 2. The minus 100-mesh fines represented 30 pct of the feed by weight. This fraction was mainly clay and earthy iron oxides.
Investigation of Process Variables
The concept in investigating the development of methods for minimizing particle segregation and improving percolation rates through clayey ores was to agglomerate the fines in such a way that the agglomerates could withstand the forces encountered during leaching and still remain permeable enough to allow favorable penetration or flow of leach solutions, as illustrated in figure 3. Lime is known to act as a flocculant in thickening and dewatering applications; and it is frequently used for protective alkalinity in conventional cyanide heap leaching. However, its use in this manner has little beneficial effect on solution flow through ore beds. It was thought possible to use burnt lime on an ore in such a way that it would react with silicates in the ore and partially set up with time, much the same as concrete does. This would involve mixing the ore with controlled amounts of lime and water and then aging for a period of time to allow hydration of the calcium silicates. Such a system might form a rigid hydrated calcium silicate bridging
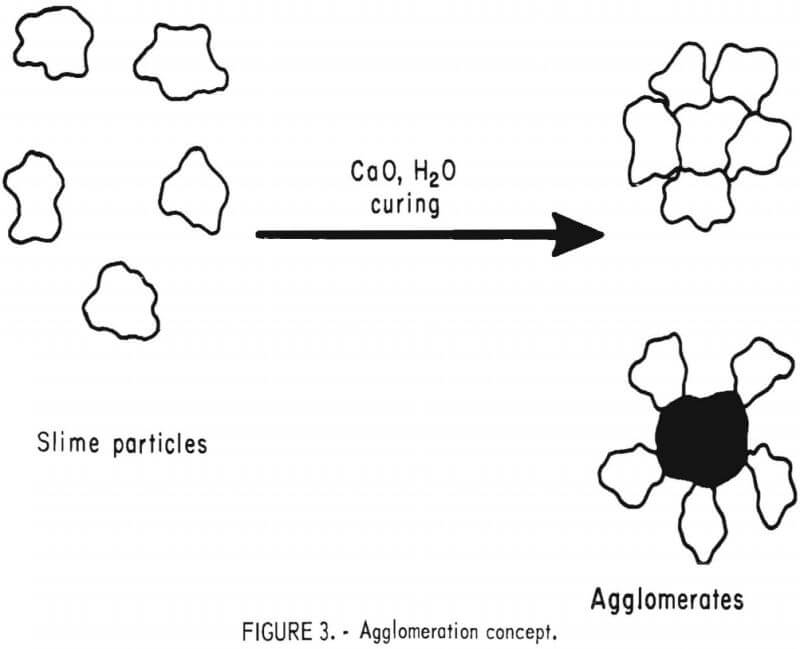
across a number of the ore fines in such a manner that the resulting agglomerate would be porous but still retain its strength during leaching. In effect then, about the same amount of lime would be added to the ore as would be ordinarily used for protective alkalinity during cyanidation, but the method of applying the lime would be modified to effectively increase the percolation rate of leach solutions.
Bench-Scale Tests on Silver Mill Tailings
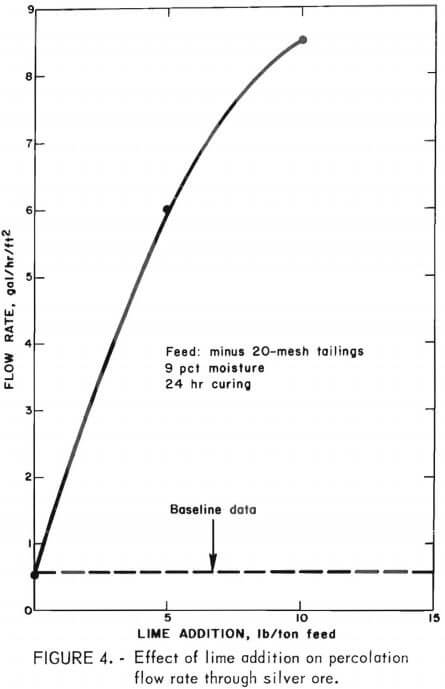
Initial column heap leach tests were conducted on silver mill tailings to obtain baseline data for comparison with data obtained in subsequent agglomeration experiments. Various amounts of lime were added to each 50-pound ore charge-not necessarily in an attempt to agglomerate the fines, but to provide protective alkalinity and obtain data on the effect of addition of lime in a conventional manner. Each experiment was conducted under identical conditions except that the amount of powdered burnt lime mixed into the 50-pound charges of dry mill tailings was varied from 0 to 30 lb/ton of feed. After addition of the dry mixture to the leach column, the downward percolation leach was started using 12 liters of solution containing 2 pounds of sodium cyanide per ton. The pregnant liquor effluent from the column was collected in a sump, then pumped upward through three columns of activated carbon in series for gold-silver recovery; the resulting barren solution was recycled to the leach column. The leach solution was recirculated through the leaching-carbon adsorption system until a steady-state flow rate had been achieved. Flow measurements were taken daily for 7 days and averaged to determine the flow rate. As expected, the results showed that the percolation flow rate was completely independent of the amount of lime added to the feed. The flow rate remained constant at 0.5 gal/hr/ft² with lime additions as high as 30 pounds of lime per ton of feed. The results are shown graphically on the baseline data in figure 4.
 Wet agglomeration techniques then were investigated to achieve particle growth or balling of ore fines using lime as a binder. The first variable studied was the effect of varying the amount of the lime additive in the agglomeration pre-treatment on the subsequent percolation flow of the leach solution through a bed of silver mill tailings. Fifty-pound batches of dry mill tailings were mixed with various amounts of burnt lime, up to 10 pounds per ton of feed. Each batch was moistened uniformly with 9 wt-pct water by mixing in a tub using a shovel. Adequate mixing was vital for the successful agglomeration of the feed. In this particular application, the fines became granulated or adhered to the surface of larger particles and thus alleviated particle segregation. The resulting product was aged or cured at ambient conditions by standing it in a covered vessel for 24 hours to minimize drying of the pellets. Increase in strength was noted with aging so long as the lime-ore mixture remained moist. If the pellets do not remain moist during the curing step, the agglomeration reaction stops and the glomerates break down upon wetting.
Wet agglomeration techniques then were investigated to achieve particle growth or balling of ore fines using lime as a binder. The first variable studied was the effect of varying the amount of the lime additive in the agglomeration pre-treatment on the subsequent percolation flow of the leach solution through a bed of silver mill tailings. Fifty-pound batches of dry mill tailings were mixed with various amounts of burnt lime, up to 10 pounds per ton of feed. Each batch was moistened uniformly with 9 wt-pct water by mixing in a tub using a shovel. Adequate mixing was vital for the successful agglomeration of the feed. In this particular application, the fines became granulated or adhered to the surface of larger particles and thus alleviated particle segregation. The resulting product was aged or cured at ambient conditions by standing it in a covered vessel for 24 hours to minimize drying of the pellets. Increase in strength was noted with aging so long as the lime-ore mixture remained moist. If the pellets do not remain moist during the curing step, the agglomeration reaction stops and the glomerates break down upon wetting.
After curing, the batch of tailings was transferred to the leach column, and downward percolation leaching was initiated. No additional reagent was required for maintaining the alkalinity of the cyanide leach solution at about pH 11 during the cyanidation treatment. Figure 4 shows that increasing the amount of lime employed improved the percolation flow rates. The curve was not extended beyond 10 pounds of lime per ton of feed, because this amount already exceeded that required to provide the typical rates of 0.2 to 3 gal/ hr/ft² of cross-sectional area applied in commercial heap leach operations that are treating readily amenable gold-silver ores. It is apparent from figure 4 that acceptable flow rates can be achieved by agglomerating the mill tailings prior to percolation leaching using lime in amounts normally required for protective alkalinity during cyanidation.
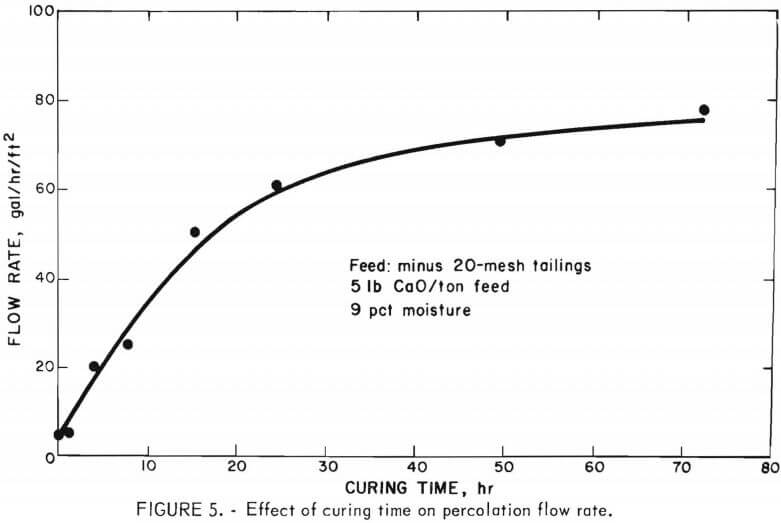
A series of column leach experiments was conducted to determine the effect of curing treatment time for the agglomerates upon the subsequent percolation flow rates. Eight 50-pound charges of the silver-bearing tailings were mixed with 5 pounds of lime per ton of feed followed by uniform moistening of the mass using 9 wt-pct water. The agglomerated charges were cured, as described previously, at ambient temperature for varying periods of time without drying. The curing periods studied were 0, 2, 4, 8, 16, 24, 48, and 72 hours. Flow rates are presented in figure 5.
The data show that the duration of the curing process or conditioning time is a key operating parameter. No improvement in percolation flow rate whatsoever is exhibited by leaching freshly prepared agglomerated feed, but the flow rates improved markedly with increasing curing time up to 20 hours and then continued to increase at a slower rate up to 72 hours of aging.
Curing of the moistened mixture of feed and lime for 24 hours resulted in a 12-fold increase in the percolation flow rate, from 0.5 to 6 gal/hr/ft².
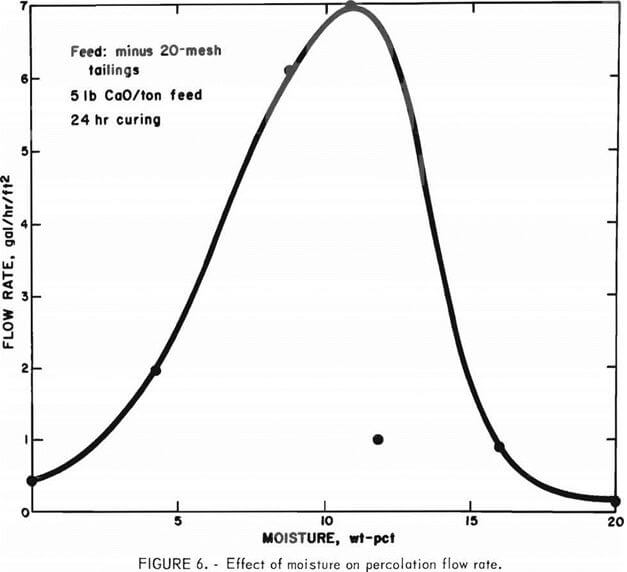
The next parameter investigated was the effect that the amount of water added to the agglomeration system exerted on the percolation rate. These experiments were conducted at constant conditions of 5 pounds of lime per ton feed and 24 hours of curing. The results are shown in figure 6.
The effects are impressive–solution flow rate through the column of tailings increased sharply with increasing moisture content, reaching a maximum of about 7.0 gal/hr/ft² at about 9 wt-pct moisture and then receding to a value of 1 gal/hr/ft² at 12 wt-pct moisture. It is apparent from these data that the permeability of the tailings is highly dependent upon the amount of water employed in the agglomeration step. If too much water is added, the tailings-lime charge becomes a mass of mud, and aging this type of material does not produce porous agglomerates.
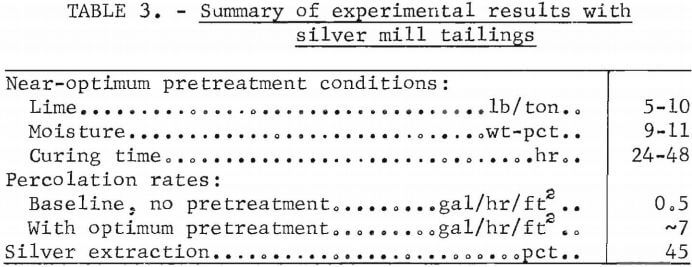
Preliminary studies showed that the conditions for effective particle agglomeration are rather critical and could, no doubt, differ markedly for other ores. The best combination of lime water, and curing time found for the minus 20-mesh tailings is summarized in table 3. Neat-optimum conditions are 5 to 10 pounds of lime per ton of feed, 9 to 11 wt pct moisture content, and 24 to 48 hours curing time. Agglomeration pretreatment increased the percolation rates from 0.5 to 7 gal/hr/ft² of cross-sectional area. Also included in table 3 is the silver extraction obtained during the simulated heap leaching sequence. The value of 45 pct extraction is essentially identical to that obtained by agitation leaching.
Bench-Scale Tests on Clayey Gold Ore
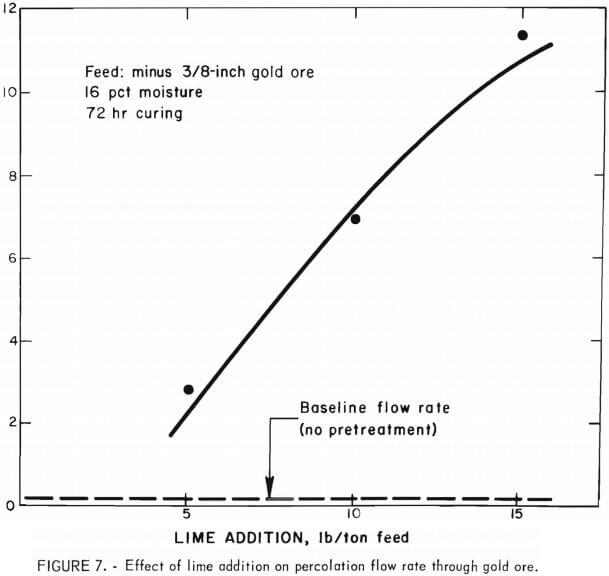
A logical extension to this work was to study the feasibility of applying the particle agglomeration technique to a clayey gold ore having poor percolation characteristics. First, baseline percolation flow rate data were obtained on ore, crushed to 3/8-inch size, to which various amounts of lime were used in conventional percolation leaching techniques. The experimental procedure was similar to that employed in obtaining baseline data for the silver-bearing tailings. The results of this series of tests also demonstrated that the amount of lime added to the ore without pretreatment had very little effect on the percolation flow rate. The flow rates obtained employing 0, 5, 10, and 15 lb of lime per ton were consistently poor, approximately 0.1 gal/hr/ft² of cross-sectional area. These baseline data are shown in figure 7 for comparison with those obtained in the subsequent agglomeration experiments.
Initial agglomeration experiments conducted on the gold ore showed that clays and earthy minerals present in the ore were very absorbent. It was impossible to achieve uniform wetting of the whole charge of ore crushed to 3/8-inch size by using the simple technique developed for the tailings.
Successful particle agglomeration was eventually carried out using a rotating disk pelletizer. Figure 8 shows a typical 50-pound charge of a dry mixture of the gold ore and lime on the pelletizer. Figure 9 shows the 50-pound ore charge after it had been agglomerated. During the treatment, the larger ore particles serve as nuclei for spherical agglomeration of the slimes and fines. Particle growth or balling occurs by the layering of clays on the surface of coarser ore fragments. The conversion of the clays or fines into large, porous, spherical particles results in an expansion of the volume of the ore charge, a more uniformly sized feed, and elimination of the adverse effect of the downward movement of the slimes. A closeup of the agglomerated material is shown in figure 10.



The effect of the amount of lime in the agglomerated feed on the percolation flow rate of cyanide leach solution was studied in a series of experiments employing 5, 10, and 15 pounds of lime per ton of ore. The ore-lime mixture was agglomerated in the pelletizer using 16 wt-pct water, and the agglomerated material was cured at ambient temperature for 72 hours in a closed percolation leach column to permit the agglomerates to develop sufficient green strength to withstand leaching without disintegration. This curing condition eliminated the possibility of adsorption of atmospheric carbon dioxide by the lime additive.
The effectiveness of increasing the amounts of lime to enhance percolation flow rate through the ore is demonstrated by the data shown in figure 7. Without particle agglomeration pretreatment, the percolation flow rate for the gold ore was only 0.1 gal/hr/ft² of cross-sectional area. It was found that the flow rate could be increased 100-fold by the particle agglomeration pretreatment. It was also observed that under these conditions no segregation or migration of the fines occurred during the percolation leaching.
The behavior of the ore charge during bedding (or addition to the column) and leaching is of interest in attempting to explain the changes in flow rate that occur with the agglomeration treatment. Figure 11 shows laboratory columns used in simulated heap leaching of treated and untreated ore. Each column contained the same weight of 3/8-inch crushed ore (50 pounds). The untreated ore (left column) is more dense and compacted than an equal weight of the agglomerated feed (right column). The bed of untreated material compacted to 38 inches during leaching, whereas the agglomerated feed compacted to only 46 inches, an 8-inch difference in the depth of the bed. Figure 12 shows that the clayey fines of the untreated ore (left column) had migrated, filling in the voids which tended to seal off the bed of ore. The right-hand column contains the leached agglomerated feed. The wetted balls of clay maintained their integrity fairly well throughout the leaching period of 5 days, and segregation of the fines was negligible.
A preliminary study was made of the application of organic flocculants in conjunction with lime to enhance the percolation flow of cyanide leach solutions. The effect of additions of polyethylene oxide (PEO) resins was investigated because they are known to act as effective flocculants for colloidal silica and clays in basic solutions. The addition of PEO in the amount of 0.1 lb PEO/ton ore increased the percolation flow rate approximately 30 pct above that obtained with lime alone (shown in fig. 7).
An attempt was made to determine the change in the nature and composition of the freshly prepared agglomerated particles and those that were aged or cured, but the preliminary results were inconclusive. Electron microscopy showed Ca 2+ cations dispersed throughout the agglomerates. However, the nature of the bridging that holds the clayey particles together was unidentifiable. We assume that particle agglomeration is similar in principle to flocculation: that is, coagulation is caused by bridging of the colloidal clay particles with the flocculating reagents and electrolytes. The function of curing in the particle agglomeration technique is to build adequate stre strength into the pellets so that they can resist disintegration during percolation leaching. The addition of PEO resin appears to enhance the bridging effect of the lime.
Process Development Unit Tests
On completion of these laboratory tests, experiments simulating heap leach cyanidation were conducted in a process development unit to compare the efficiency of the particle agglomeration method with the conventional practice as they relate to flow rates and gold recovery.
Three tons of the clayey gold ore were crushed to a nominal 1-inch size and split into two equal portions. In the baseline test, 1.5 tons of crushed ore was mixed with 5 pounds of lime per ton of ore and placed in the PDU leach column described earlier. This volume of ore upon wetting made a bed 10 feet deep in the 24-inch-diameter column. A cyanide solution containing 1.0 pound of NaCN per ton was employed for leaching. A flooding method was chosen, by necessity, for leaching the untreated ore in the baseline test because the ore had very poor percolation characteristics.


The system that gave the best flow rates in laboratory tests was employed in the particle agglomeration test. The ore was mixed with 15 pounds of lime per ton and then agglomerated using a solution containing 0.1 pound of PEO per ton of ore to moisten the feed. The amount of solution employed was 12 wt-pct. Less solution was required because the 1-inch feed contained fewer fines than the 3/8-inch feed used in the laboratory experiments. The agglomerated material was placed in the leach column and aged for 72 hours. The leach solution was introduced onto the ore charge by a mechanized rotating distributor that discharged the solution through two perforated pipes to produce large droplets. The top of the bed of ore was covered with coco matting to prevent erosion of the agglomerated ore particles by impingement of the solution. The pump was regulated so that the flow did not build a head of leach solution on top of the ore charge. The gold and silver values were recovered from the pregnant effluents in both tests by adsorption on activated carbon. Barren cyanide solutions were recirculated to the leach column.
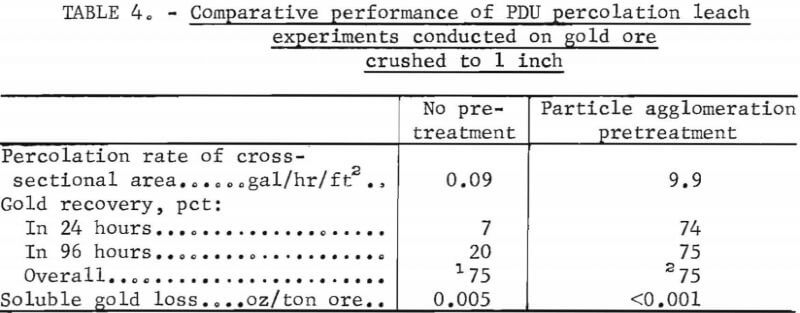
Table 4 compares results obtained in the baseline experiment on unagglomerated ore with those obtained in an experiment on agglomerated ore. The data show that the time required to reach equivalent gold extraction with the agglomerated ore was about one-thirtieth of that required with untreated ore and that soluble losses with the agglomerated ore were almost nil, whereas those encountered with the untreated ore were 0.005 ounce per ton of ore. The results are highly encouraging in the light of the fact that the conditions employed in conducting the experiments were idealized compared to those usually encountered, in actual field operations, wherein considerable particle segregation would be expected to occur during preparation of the heap for leaching.
Potential Benefits Using Agglomeration Techniques
Several potential benefits can be attributed to particle agglomeration pretreatment of ores prior to heap leach operations:
- Clayey and finely crushed ores are rendered amenable to low-cost heap leaching procedures. This would allow treatment of large bodies of low-grade ores, many of which are presently considered submarginal.
- Increased overall gold recovery from an ore may be obtained because additional gold could be liberated by finer crushing without encountering particle segregation during preparation of ore heaps. Particle segregation can cause localized accumulations of fines that inhibit flow of leach solutions.
- Percolation flow rates are increased, thus decreasing the required leaching time. This could have economic implications in that the mine capacity could be increased without significantly increasing capital cost for pad preparation.
- The highly porous nature of the agglomerates would allow the heaps to “breathe,” thereby providing the oxygen necessary for the gold dissolution reaction. This could allow elevation of the height of the heaps so that pad preparation cost per ton of ore processed would be less and land area could be more effectively utilized.
- Favorable environmental implications may be realized in that the highly porous structure of the heap would allow efficient washing of residual cyanide from spent ore heaps. Dusting problems from abandoned heaps of the stable agglomerates should be minimized.
- Precious metal concentration in solutions exiting from the heaps should be higher, which could be expected to enhance efficiency of gold silver recovery from these solutions by precipitation or adsorption on activated carbon.
Research is being continued to further develop improvements in the agglomeration of clayey gold-silver ores to advance the technology for heap leach cyanidation processing of low-grade resources. A variety of binders or bonding reagents are being evaluated both with and without alkaline compounds to provide protective alkalinity during cyanidation. Cursory studies of magnesia, calcined dolomite, calcium chloride, and portland cement (type II) have been initiated. Of these, portland cement has the most practical significance. Cement additions of 5 to 10 pounds per ton of clayey ore used in this investigation produced exceptionally stable agglomerates with high porosity. The resulting agglomerates endured percolation leaching using spray or flooding methods to simulate heap and vat leaching, respectively, with minimal deterioration of the feed material.
Heap Leaching Gold Silver Ores
Solution flow rates through ore heaps are effectively increased by agglomerating fine particles in the ore with lime, water, and an aging or curing step. Portland-type cement can be used in conjunction with or substituted for the lime. The agglomeration procedure solves the problem of particle segregation during the stacking of crushed ore and results in more rapid recovery of the precious metal values, lower soluble gold and silver losses, and more thorough washing of the ore which may significantly lower the content of residual free cyanide in leached ore heaps. By improving percolation leaching, these techniques have the potential of helping to solve environmental problems associated with tailings disposal as well as making an important economic impact on gold-silver recovery operations.
To maximize productivity through improved minerals processing technology, the U.S. Department of the Interior, Bureau of Mines, is investigating particle agglomeration techniques as a means of increasing the percolation rates of leach solutions in heap leaching of clayey gold-silver ores. Simulated heap leach experiments were conducted on 50-pound charges of ore that were contained in 5.5-inch-diameter columns, 5 feet in height. Results indicated that the percolation rate of cyanide leach solution could be enhanced markedly by mixing the ore with controlled amounts of lime, flocculants, and water, and then aging the mixture prior to the column leach step. Experimental results obtained on 20-mesh silver tailings and gold ore crushed to 3/8 inch and to 1 inch showed that it was possible to increase percolation rates as much as 100-fold without sacrificing silver-gold extraction. Larger scale tests conducted in 24-inch-diameter, 16-foot-high columns closely paralleled bench-scale results. By improving percolation leaching, these techniques have the potential of helping to solve environmental problems associated with tailings disposal as well as making an important economic impact on gold-silver recovery operations. Investigations are continuing to develop binders superior to lime for particle agglomeration pretreatment.
