Table of Contents
- Fluidized-Bed Roasting of Chromium Sulfide
- Reactor Design
- Effect of Water Content
- Effect of Initial Particle Size
- Aluminothermic Reduction to Chromium Metal
- Desulfurization
- Leaching Nickel Scrap
- Batch Chlorine Leaching Tests
- Effect of Copper Activation
- Effect of Redox Potential Control
- Copper Removal
- CCD Countercurrent Leaching System
- Raffinate Purification
- Continuous Leaching Tests
- Cleaner Leach of Chromium Concentrate
- Direct Leaching of Superalloy Matte
- Cobalt Extraction from Scrap Alloy
- Cobalt Solvent Extraction
- Electrowinning
- Recovery of Metals
Fluidized-Bed Roasting of Chromium Sulfide
The chromium concentrate produced either directly as flotation tails or indirectly as a leach residue is composed primarily of chromium sulfide with chromium comprising about 80 percent of the metal content. Nickel and molybdenum sulfides would be present in the flotation tails along with heavy metal oxides. The leach residue would also contain a substantial quantity of elemental sulfur which would be removed in a commercial operation by flotation.
The next step in the production of metallic chromium is removal of combined sulfur by roasting in a highly oxidizing environment. Fluidized-bed roasting was selected for this operation because it provides excellent contact between the product and the reacting gas and hence good operating efficiency. The process is applied on a commercial scale to the roasting of nickel sulfides; hence experience with this system may be applicable to the present problem.
Chemical Reactions
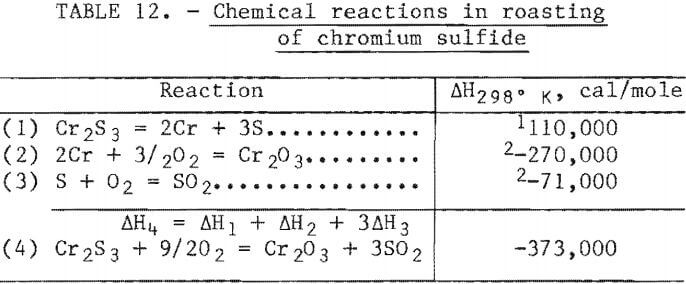
The reactions that would be expected in a reactor treating a slurried feed of chromium sulfide are listed in table 12. The heat of reaction of the Cr2S3 phase has apparently never been determined. Consequently this quantity was estimated from information available on related chromium sulfide compounds (9, 18, 24). For roasting a pure chromium sulfide the overall reaction is clearly exothermic. The actual chromium sulfide phase in the concentrate has Ni and small quantities of Co, Fe, and Mo atoms substituted for Cr atoms. The basic oxidation reaction does not change, but the heat evolved in the reaction is approximately 10 percent lower than that of the pure chromium sulfide.
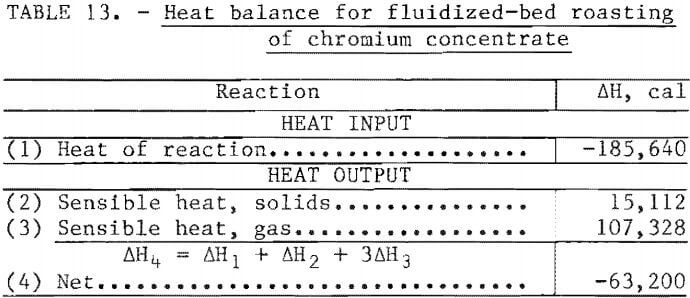
The heat balance for a fluidized-bed roaster using this feed can be estimated provided certain assumptions are made. The heat balance shown in table 13 was derived using the assumptions listed in the table. The heat losses are obviously a function of the size and efficiency of the roaster and may be as low as 1 percent of the heat of reaction in a large commercial-scale roaster. Based on experience in operating this type of system, a roaster handling more than a few kilograms per hour should be self-sustaining. The analysis presented in table 13 assumes an oxidation efficiency of 90 percent. If this estimate is correct, the exit gas will contain approximately 11 mole- percent SO2 which must be removed by wet scrubbing before venting to the atmosphere.
Assumptions:
- Analysis is based on 100 g of feed (dry basis).
- Feed is a slurry of chromium sulfide (M2S3) containing 15 wt-pct water.
- M represents a metal atom with a ratio 4Cr:1Ni.
- ∆H for M2S3 and M2O3 are 10 pct lower than the ∆H for the pure chromium compounds.
- Feed enters the reactor at 20° C and exits at 1,073° C.
- Gas enters the reactor at 20° C and exits at 1,073° C.
- The oxidizing gas is air, and the oxidizing efficiency is 90 pct.
- Exit gas composition is mole-pct: N2-78, SO2-13, H2O-13, H2O-7, O2-2.
- Heat capacity of gas mixture calculated from data in reference 18 as 8.2 cal/deg-mole.
- Heat capacity of solids calculated from data in reference 18 as 27.6 cal/deg-mole.
Experimental Procedure
Although the roasting process should be thermally autogenous on a pilot- or commercial-scale reactor, this is not the case for a reactor suitable for handling the small quantities of material available in the laboratory program. Consequently, reactor design and experimental objectives had to take this limitation into account.
Reactor Design
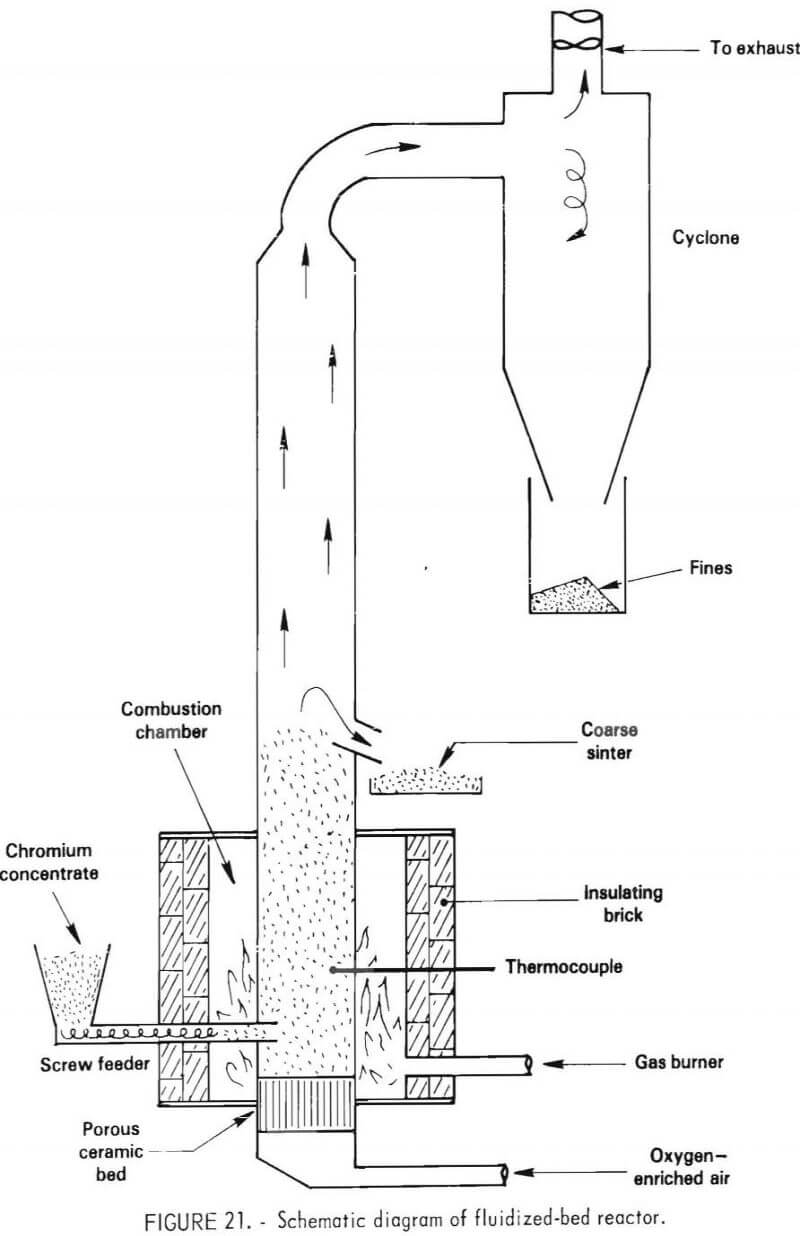
The configuration of the apparatus used for fluidized-bed roasting evolved over the period of the testing program, as operating problems were identified. Only the final, most efficient design will be discussed. The schematic diagram of the apparatus is shown in figure 21. External heating was provided by a gas torch in the annular heating chamber. The reactor was fed by a motor-driven screw feeder which introduced a slurry of ground matte or flotation concentrate containing 5 to 10 percent water. The feed rate was approximately 20 grams per minute. Cold reaction gas was fed at a rate of 150 cubic feet per minute into the bottom of the reactor through a ceramic honeycomb. The fluidizing gas was oxygen-enriched air. The bed height was approximately 12 inches with a product discharge into an open container. The gaseous products were passed through a cyclone to remove dust and then vented to the foundry offgas treatment system.
Raw Materials
Considerable difficulty was experienced in obtaining a feed for the roaster which was truly representative of that expected for the unified flowsheet. This was largely the result of the difficulty in separating nickel sulfide fines in froth flotation as discussed earlier. A portion of the feed was minus 200-mesh blended product of a number of flotation tests containing approximately 35 percent Ni, 20 percent Cr, and 35 percent S. A few tests were also conducted using high-sulfur matte ground to minus 40 mesh and concentrated by magnetic separation. This material contained 45 percent Ni, 15 percent Cr, and 33 percent S.
Results
Because of the small size of the reactor, it was difficult to operate in a continuous mode. Use of the screw feeder greatly improved particle circulation within the reactor; however, it was not possible to fully eliminate channeling. As a consequence, the sulfur content of the bed was inhomogeneous during the initial stages of a run. This inhomogeneity precluded accurate sampling to develop information on kinetics of desulfurization. Consequently, the variables discussed in the subsequent sections were evaluated on the basis of the final bed composition.
Effect of Water Content
It was found that a reaction temperature of 1,800° to 2,200° F was needed to achieve desulfurization. This temperature is higher than the melting point of the sulfide; consequently rapid fusion of the charge occurred when dry concentrate was fed into the reactor. This problem was solved by blending the feed with 5 to 10 percent water. The actual mechanism by which water solves this problem is not known, but it is presumed that water promotes more rapid surface oxidation, which in turn prevents formation of agglomerated particles too large to fluidize.
Effect of Initial Particle Size
Limited testing showed that feed particle size was very important. The minus 325-mesh flotation concentrate was desulfurized within 15 minutes at 2,000° F, while minus 40-mesh ground matte required 4 hours. The feed for a commercial plant would, of course, resemble the flotation concentrate.
Minimum Final Sulfur Content
Although rapid desulfurization to 2 percent sulfur could be achieved, further reduction as required for a product for the superalloy industry took considerably longer owing to instability of the system. The lowest sulfur level achieved by direct roasting was 0.23 percent. When this material was water-agglomerated and reroasted, a product containing 0.022 percent sulfur and 21 percent oxygen was obtained.
Discussion
The roasting studies described above demonstrated the feasibility of achieving low sulfur levels in a chromium flotation concentrate despite the difficulties associated with operating the small system. With a more stable bed, it should be possible to achieve the 0.005-percent sulfur levels desired for the product. The chilling effect of the inlet gas contributed to the excessive roasting times experienced in the laboratory experiments. A gas preheating system (designed to utilize the sensible heat of the exhaust gas) would be desirable to improve the efficiency of the reaction system. Although other roasting methods could be applied, based on past experience in roasting nickel sulfides it appears that the fluidized-bed system would be the most efficient and provide the most desirable product for aluminothermic reduction.
Aluminothermic Reduction to Chromium Metal
Two methods are used commercially to produce pure chromium metal: Aluminothermic reduction and electrowinning. Both processes are capable of generating a product of purity sufficient for use in the superalloy industry.
The reason for selecting the former process is that the fluidized-bed roaster product contains nickel and possibly some heavy metals along with chromium. Production of chromium by electrowinning requires an electrolyte of relatively high purity, and hence a complex hydrometallurgical purification circuit would be needed. However, aluminothermic reduction can be used directly on a mixed metal feed. Since a chromium product destined for use in superalloys could tolerate a significant quantity of nickel, aluminothermic reduction was a logical choice. The basic reduction process is well known; however, it has not generally been applied to a derivative of a sulfide intermediate. Consequently, efficiency of desulfurization was a factor in the experimental demonstration as well as overall chromium recovery.
Chemical Reactions
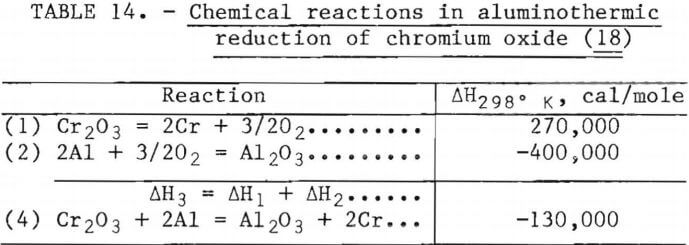
Aluminothermic reduction depends upon the heat of reaction of the products being greater than the heat of reaction of the reactants. These reactions must be highly exothermic so that the reaction products are not only molten but sufficiently fluid to facilitate separation of metal and slag. The reactions for this system are listed in table 14.
The charge for a metallothermic reduction system is generally cold. Since fluxes are also added to promote a fluid slag, the reaction must be sufficiently exothermic to melt the entire charge. A heat balance for a hypothetical reaction is shown in table 15, along with the assumptions used. The heat evolved is sufficient for melting the reaction products from a chromium concentrate charge having a 4Cr:1Ni ratio. Although there is a surge of dust during the brief reaction period, there are no gas-producing reactions; thus only dust removal is required for treating the exhaust gas from this type of reactor.
Experimental Procedure
Metallothermic reductions are conducted on a batch basis. As noted above the process can be carried out on rather small charges provided the reaction is sufficiently exothermic. Consequently, most of the problems of scale encountered with fluidized-bed roasting did not apply to the reduction tests.
Raw Materials
The feed for these tests consisted of roasted chromium concentrate or pure metal oxides blended to provide a composition similar to that expected for an integrated flowsheet. This material was mixed with aluminum powder (reductant) and lime and fluorspar (fluxes).
Testing Procedure
The reaction vessel consisted of a clay-graphite crucible backed with foundry sand and contained in a steel shell. The oxide charge weight ranged from 0.52 to 1.0 kilograms. The quantities of aluminum reductant and flux were also varied. Emphasis was placed on defining a reductant and flux combination that would provide maximum chromium recovery in the metal and maximum sulfur recovery in the slag. The procedure, which was used for all of the tests, was to preheat the crucible with a gas torch to about 1,200° F. A plastic bag containing the charge was dropped into the crucible and ignited with the torch. The metal and slag were allowed to solidify in the reaction vessel.
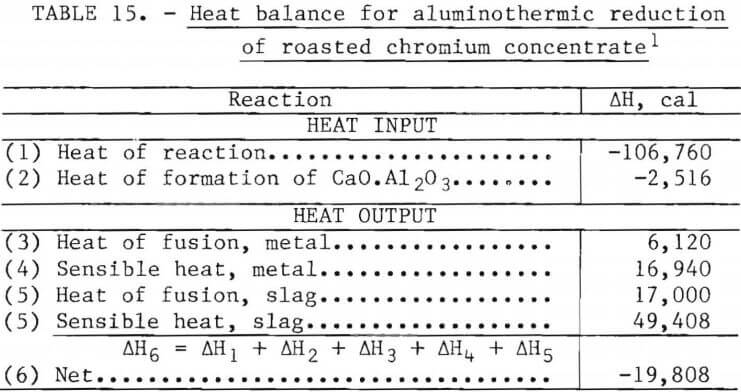
Assumptions:
- Analysis is based on 100 g of dry chromium concentrate.
- Feed is roasted chromium concentrate (M2O3), lime (CaO), and metallic aluminum powder.
- M represents a metal atom with a ratio of 4Cr:1Ni.
- Slag is CaO.Al2O3.
- ∆H for M2O3 is 10 pct lower than ∆H for Cr2O3.
- ∆H for CaO.Al2O3 is -3,700 cal/mole.
- Reactants are charged at 20° C.
- Products reach a maximum temperature of 1,750° C at which point both metal and slag are fully molten.
- Reduction is 100 pct efficient.
- Heat of fusion of alloy estimated from reference 18 is 4,500 cal/mole.
- Heat of fusion of slag estimated from data on related compounds from reference 18 is 25,000 cal/mole.
- Specific heat of alloy calculated from data in reference 18 as 7.2 cal/deg-mole.
- Specific heat of slag calculated from data in reference 18 as 42.0 cal/deg-mole.
Results
General Observations
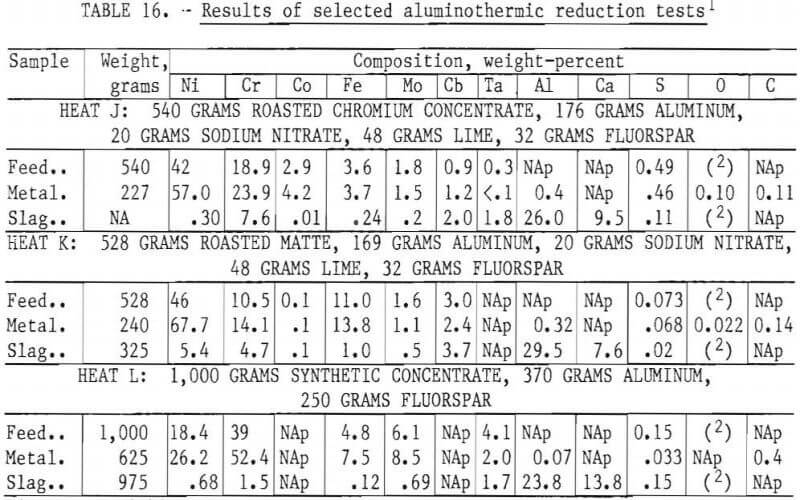
Seven aluminothermic reduction tests were performed. In general the reactions were relatively mild and controlled, each lasting about 20 seconds. Complete melting and separation of the metal and slag were achieved in every case. The slag could be easily separated from the metal on removal from the crucible as there was no interpenetration during solidification. No crucible reaction were observed. The results of selected tests are shown in table 16. Based on these results, it appears that virtually all of the Cr, Ni, Co, Fe, and Mo contained in the charge report to the metal ingot. Columbium and tantalum, when present in the feed, were distributed in both metal and slag phases. Recovery of aluminum in the metal was very low.
Desulfurization
The sulfur content of the metal ingot should be less than 0.010 percent, and preferably less than 0.005 percent, if it is to be acceptable for use in superalloys. The partitioning of sulfur during an aluminothermic reaction depends largely on the character of the slag formed from the reaction products. An acid slag will not hold much sulfur and, as a result, the sulfur content of the metal product will be about the same as that of the charge, as illustrated by heats J and K in table 16. The basicity of the slag can be increased by using lime, magnesia, fluorspar, or other additives. The effectiveness of this approach is shown by the results of heat L, in which the sulfur content was lowered from 0.15 percent to 0.033 percent. Although the target sulfur level of 0.005 percent was not achieved, the initial sulfur level in the charge was quite high. A roaster product having a sulfur level of 0.02 percent, which was achieved in previous trials, should provide a final product with the desired sulfur content.
Material Balance
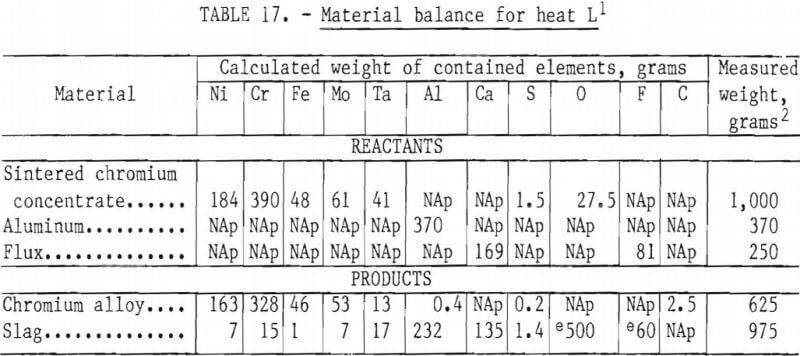
A material balance was prepared for heat L. The information is shown in table 17. For this test only 20 grams, or 1 percent of the charge weight, could not be accounted for. It is assumed that this material was lost as dust. The individual element recoveries do not agree quite so well, primarily because of the slag analyses, which appear low. The metal recoveries in this test were Cr—85 percent, Ni—90 percent, Fe—98 percent, Mo—90 percent, and Ta—32 percent. Distribution of sulfur was 13 percent in the metal and 87 percent in the slag.
Discussion
The restricted testing program that was possible with the limited amount of feedstock available demonstrated that the aluminothermic reduction operation was feasible and appropriate for producing a useful melting ingot. Further optimization of the operation in process scale-up would provide higher recovery of the metals in the ingot through more efficient utilization of heat and slower freezing. Efficiency of desulfurization would also be improved.
As noted above, recovery of columbium and particularly tantalum in the ingot was poor. Since the concentration of these elements is likely to vary considerably in the initial feedstock, it is difficult to predict typical levels in aluminothermic reduction slags. However, it is assumed that when the tantalum content of the slag is sufficiently high, recovery through additional slag treatment would be possible. Investigation of tantalum recovery from slag was considered to be beyond the scope of this investigation.
Leaching Nickel Scrap
This portion of the flowsheet involves leaching of the nickel-rich metallic concentrate from magnetic separation and the nickel-sulfide flotation product. The basic chlorine leach system has been used for leaching nickel mattes and metallic superalloy scrap. This system was adopted for its rapid leaching kinetics and selectivity and the compatibility of chloride solutions with cobalt solvent extraction systems. The leaching circuit also involves separation of the raffinate from the leach residue, hydrolysis to remove Fe, Mo, and Cr, and filtering of the residue. The resulting purified nickel-cobalt liquor proceeds to solvent extraction.
Raw Materials
A variety of materials were used in the leaching studies. Most of the batch tests and all of the continuous leaching tests were performed on material obtained from the 40-kilogram heats made from superalloy scrap. The actual samples used for leaching were a composite of the cleaner magnetics fraction produced in the separation testwork. The head feed analysis reported below for the leaching studies represents the blended fractions and thus does not correspond to the composition of the product of optimum test conditions.
A limited number of batch leaching tests were performed on other feed material. These included a sample of cleaner magnetics containing tungsten, various samples of ground, unseparated matte, and a sample of chromium concentrate.
Batch Chlorine Leaching Tests
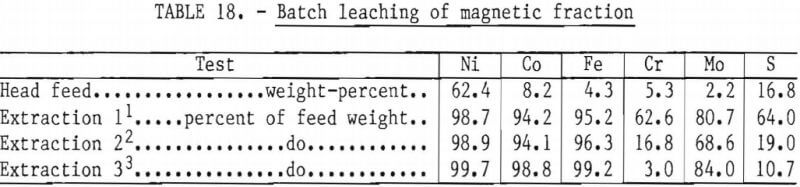
The initial testwork was carried out on direct leaching of magnetic concentrate in 1-liter batches. The analysis of a typical sample head feed is shown in table 18. The test program was performed in baffled beakers with vigorous agitation. The first few tests were performed at 80° to 95° C with chlorine as the oxidant and various mixtures of HCl, water, and nickel chloride as the lixivant. The results showed that the leaching rate was very slow; leaching times of 10 hours were required for 98-percent extraction of nickel.
Effect of Copper Activation
Based on prior experience in chlorine leaching of nickel mattes, the effect of copper was investigated as a means of increasing the leaching rate. Copper was added as cupric chloride to make a synthetic spent electrolyte solution containing 80 grams per liter of nickel and 60 grams per liter of copper. Copper is believed to act as a chlorine carrier through the operation of the cuprous-cupric ion couple. The effects of copper on leaching of superalloy-derived matte were dramatic: the leaching rate was increased by an order of magnitude. In addition, it was observed that oxidation of sulfur to sulfate and leaching of chromium were significantly reduced and chlorine efficiency was improved. This is desirable, as sulfate must ultimately be removed from the system, while the dissolved chromium makes subsequent purification by hydrolysis more difficult. The effect of the copper addition is illustrated in table 18.
Effect of Redox Potential Control
It was observed that, as chlorine was added during the leach and the redox potential rose, chromium extraction and sulfur oxidation both increased. Additional tests showed that, if the leach was stopped at a potential of about +550 millivolts (platinum versus standard calomel electrode), almost complete extraction of nickel and cobalt were achieved with very little extraction of chromium. The results of a 1-hour batch leach with redox potential of +560 millivolts are shown in table 18. This observation of selective leaching of nickel and chromium sulfides has a number of implications for the overall recovery process, which are discussed in the discussion section for the leaching experiments.
Copper Removal
When copper is used for leaching, a means for copper removal and recycling must be provided. One method that was tried was direct cementation using the raw magnetics concentrate. This approach was not successful, and very little copper was precipitated. Previous experience has shown that cementation can be improved through the use of elemental sulfur, which promotes precipitation of copper sulfide as discrete particles rather than metallic copper films which coat the matte particles and impede the reaction. The addition of sulfur in the atomic ratio of four sulfur to one copper was examined in a batch test. In this test a solution containing 148 grams per liter of nickel and 4.8 grams per liter of copper was contacted with 180 grams per liter of magnetics feed and 1.9 grams per liter of elemental sulfur. After 30 minutes the solution contained 158 grams per liter of nickel and only 0.12 gram per liter of copper.
CCD Countercurrent Leaching System
Based on the results of the preceding tests, a countercurrent leaching scheme was proposed to facilitate recirculation of copper within the leaching system. This scheme is illustrated in figure 22. In this system, the feed is initially contacted with the filtrate solution containing copper. Partial leaching is accompanied by copper cementation. The slurry is then pumped to a thickener, the overflow of which constitutes the pregnant liquor for nickel and cobalt recovery. The underflow, about 90 percent of the original leach feed, goes to the completion leach. In this step spent electrolyte returning from electrowinning is used with chlorine under controlled redox potential to extract as much nickel and cobalt as possible without leaching chromium. The copper sulfide dissolves during this step. The effluent is filtered to remove the residue, mainly sulfur and chromium sulfide, and returned to the cementation leach.
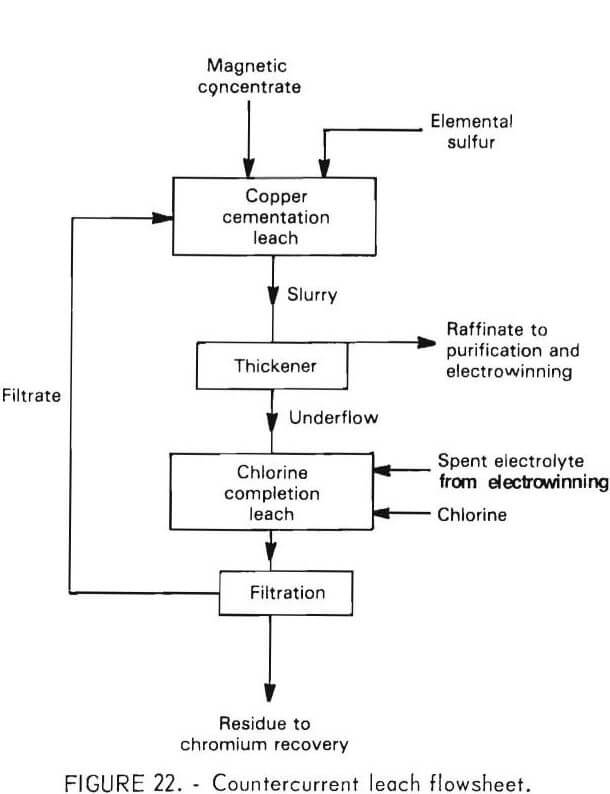 The proposed system was tested using a batch simulation which showed that it was workable. The system was then adopted for the scale-up of continuous leaching.
The proposed system was tested using a batch simulation which showed that it was workable. The system was then adopted for the scale-up of continuous leaching.
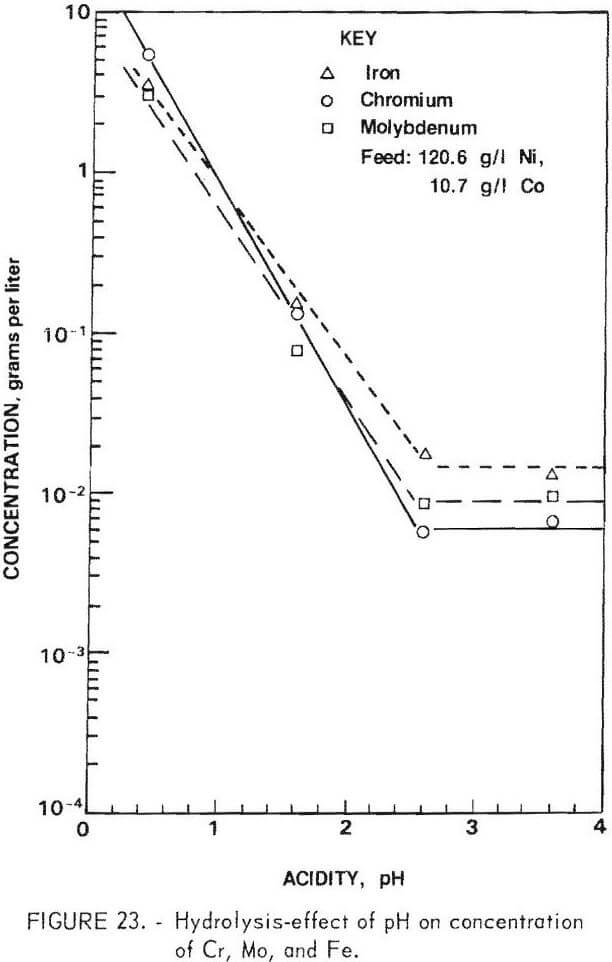
Raffinate Purification
As shown in table 18, the chlorine leach also extracts Fe and Mo, along with Ni, Co, and a small amount of Cr. The separation of nickel and cobalt cannot be performed until other elements are removed from solution. This can be accomplished by pH adjustment which precipitates Fe, Mo, and Cr as hydroxides. Nickel carbonate was a logical choice because contamination of the system was thereby avoided. The effectiveness of the hydrolysis procedure is demonstrated by the results shown in figure 23. The precipitate formed during hydrolysis was somewhat difficult to filter. Tests showed that, when high chromium extractions were achieved, the nickel losses to the filter cake became excessive. In the test cited above, the precipitate contained 17 percent Fe, 13 percent Cr, 10 percent Mo, and 10 percent Ni. About half of the latter was recovered by washing, giving a total nickel and cobalt recovery in this operation of 97.5 percent.
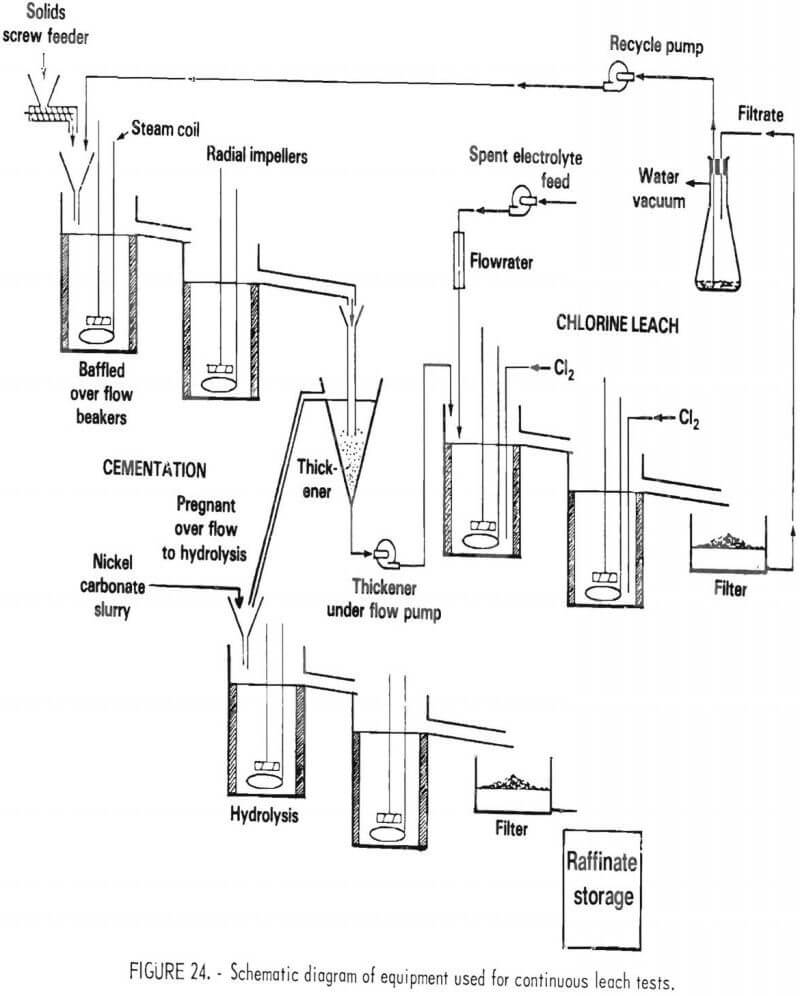
Continuous Leaching Tests
An apparatus was set up to conduct countercurrent leaching and hydrolysis on a continuous basis. A schematic diagram of this system is shown in figure 24. The vessels, which had a 1.5-liter operating volume, were maintained at 80° C using titanium steam coils. Titanium agitators and baffles were employed. Chlorine was added to the leach tanks under redox control with solenoid-actuated valves made of Teflon controlling the chlorine flow.
 The first leaching vessel was controlled at +400 millivolts and the second at +550 millivolts. A glass
The first leaching vessel was controlled at +400 millivolts and the second at +550 millivolts. A glass
separatory funnel was used as a thickener.
Results of First Continuous Test
The results of the first eight-hour test are contained in table 19. Operating problems were encountered during the test; however, acceptable nickel and cobalt extractions were obtained (97.5 and 94.9 percent, respectively). Copper cementation was irregular owing to variable flow rates, and a buildup of particles in the cementation leach vessels made it difficult to maintain the desired copper level in the completion leach vessels. One encouraging sign was that chromium extraction and sulfate formation were both low, indicating the effectiveness of redox potential control. The hydrolysis system worked quite well with very low and stable levels of Fe, Cr, and Mo in the filtrate. The hydrolysis slurry was filtered at a rate of 1,500 liters per square meter per hour. Washing effectively removed the entrained nickel and cobalt. Under stable operating conditions at the end of the test, the nickel and cobalt losses to the filter cake were 0.3 and 2.1 percent, respectively.
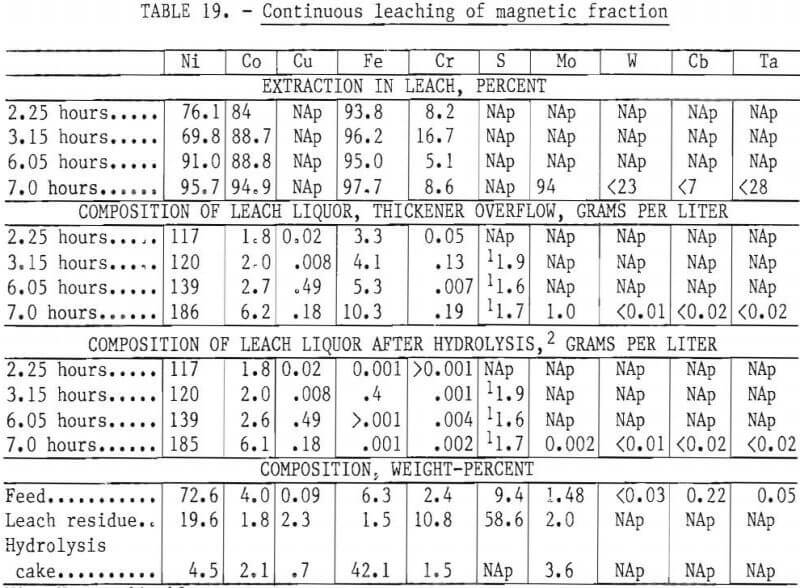
The distribution of the heavy metals Mo, W, Cb, and Ta was assessed at the end of the run. The results indicate that the latter three elements were not dissolved in the chlorine leach and reported to the leach residue. Very high extraction of molybdenum was achieved, along with virtually complete precipitation during hydrolysis. Consequently, most of the molybdenum in the feed was captured in the hydrolysis filter cake.
Results of Second Continuous Test
Because of the difficult in pumping the coarse feed particles through the system, the feed for the second test was screened to minus 85 mesh. Although solids buildup was eliminated, uneven flow produced a large variation in the copper concentration of the final leach liquor (from 0.1 to 1.0 gram per liter). Subsequent batch tests showed that copper levels of <10 milligrams per liter could be achieved and thus should be realized in large-scale operation where the flow can be more readily controlled.
Cleaner Leach of Chromium Concentrate
Examination of the primary leach residue showed it to consist of a mixture of chromium and heavy metal sulfides and elemental sulfur. This indicated that under redox control, chromium sulfide was practically unleached. Physical separation of matte produced and chromium concentrate which contained a significant amount of unfloated nickel sulfide, along with nickel dissolved
in chromium sulfide. In an effort to further concentrate chromium, a sample of flotation tails was given a chlorine leach at a controlled redox potential of +550 millivolts in the presence of copper. In this small batch test, the concentrate was upgraded from a chromium-nickel ratio of 0.48:1 to 1.08:1. Chromium extraction was 9.3 percent (results listed in table 20). The test conditions were not optimized for this feed; however, the results indicates that it is feasible to leach nickel sulfide from the concentrate. It is assumed that nickel dissolved in the chromium sulfide will not leach, and hence the theoretical best chromium-nickel ratio that can be achieved is 4:1.
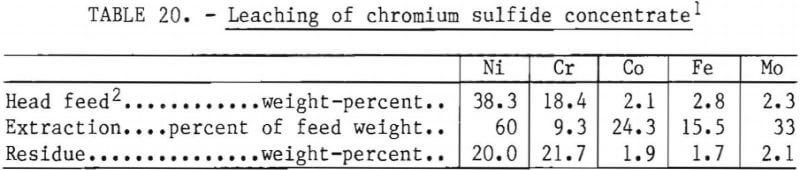
Direct Leaching of Superalloy Matte
The results of the preceding test suggested that it might be possible to directly leach the superalloy matte, recover most of the nickel and cobalt, and reject chromium to the leach residue, where it could be recovered without going through the inefficient magnetic and flotation steps. To test this idea, several batch tests using a range of leaching conditions were conducted on samples of freshly ground matte. The results of the best test are shown in table 21. The separation of nickel and chromium was extremely good with the residue chromium-nickel ratio approaching the theoretical 4:1. Only limited experimental work was done on the direct leaching approach, since its feasibility was not realized until the very end of the testing program. However, on the basis of the results, it offers the possibility of greatly simplifying the metal recovery process.
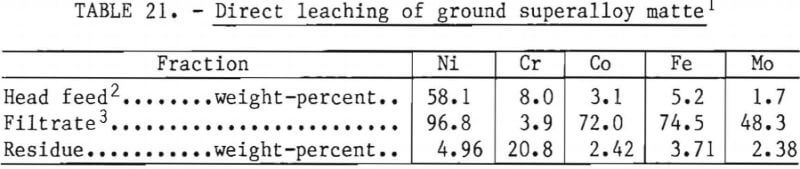
Discussion
The results of the leaching experiments clearly established the technical feasibility of this portion of the recovery flowsheet. The observation that a nickel-chromium separation could be made through controlling the leaching conditions was not anticipated at the outset of the program. Consequently, it was not possible to fully explore the implications of this separation within the time limitations. In view of the limited amount of data available on the direct matte leach procedure as well as flotation separation, both schemes require more study to determine the more acceptable approach.
It was not possible in the laboratory work to do more than a cursory evaluation of the effect of matte composition on leaching. A number of questions would have to be resolved at a pilot plant stage or in further laboratory studies. In particular, the effect of iron content should be examined in detail. As noted earlier, when the iron level in the feed reaches 20 percent, iron-chromium sulfides are formed. The behavior of this sulfide in the leach is unknown. Iron is also the major constituent in the hydrolysis filter cake, and difficulties in handling this cake and attendant nickel and cobalt losses would be expected to increase with increasing iron content. Copper is also a frequent constituent of nickel alloy scrap. A high copper level in the feed could cause an excessive buildup of copper within the leaching circuit. Finally, the leaching behavior of the many trace elements known to affect the properties of superalloys must ultimately be determined.
The character of the two filter residues is critical to the success of the leaching scheme. The leach residue contains essentially all of the chromium plus heavy metals and elemental sulfur. Filtering of this residue was relatively easy. In a commercial operation, the sulfur would be removed by flotation, dried, and recycled to the melting step. The remaining portion of the primary leach residue would be blended with the flotation tailings or the residue from a chromium concentrate cleaner leach to form the feed for fluidized-bed roasting and chromium recovery.
The residue from the hydrolysis operation presents more difficulty in filtering and must be carefully washed to avoid nickel and cobalt losses. The components of the residue are primarily iron and molybdenum hydroxides. The results of the laboratory work indicates that between 30 and 70 percent of the molybdenum present in the scrap feed will report to the residue. Factors that affect molybdenum recovery at this stage would include sulfur content of the matte and the initial molybdenum level. Because of its intrinsic value, it is important that the hydrolysis residue be treated for molybdenum recovery. Treatment to separate the metals from the cake could be done using established technology and hence was not investigated in the program.
Cobalt Extraction from Scrap Alloy
Following hydrolysis, the leach liquor contains only Ni, Co, and possibly small quantities of Cu and sulfate. Since the objective of the recovery process was to produce metal products that would be widely useful for superalloys, electrowinning from solution to pure metals was selected. Although a number of procedures for extracting cobalt from nickel solutions are known, solid-liquid ion exchange and liquid-liquid solvent extraction appeared most suitable for this application. The feed for the initial batch tests was synthetic, while in later tests purified raffinate from continuous leach experiments was employed.
Ion Exchange Tests
Cobalt loading isotherms were developed for three strong base and two weak base resins. Amberlite IRA-400 (quaternary ammonium-gel type resin) gave the best distribution ratio for a synthetic raffinate containing 189 grams per liter of nickel and 9.9 grams per liter of cobalt. This system was then tested in a fixed-bed column containing 1.21 liter of resin. Loading characteristics were excellent, and the cobalt concentration of the effluent averaged 5 milligrams per liter. Unfortunately, despite the good extraction, very poor cobalt-nickel separation was obtained during elution with 0.01-normal hydrochloric acid. The eluate contained 78 grams per liter of cobalt and 47 grams per liter of nickel. Because of this poor separation, additional eluate treatment would be required with this system.
Cobalt Solvent Extraction
Efficient extraction of cobalt from a chloride solution using tertiary amine triisooctylamine was reported by Brooks. Although it was known that phase separation was somewhat difficult with this type of system, it was felt that better separation of nickel and cobalt could be obtained.
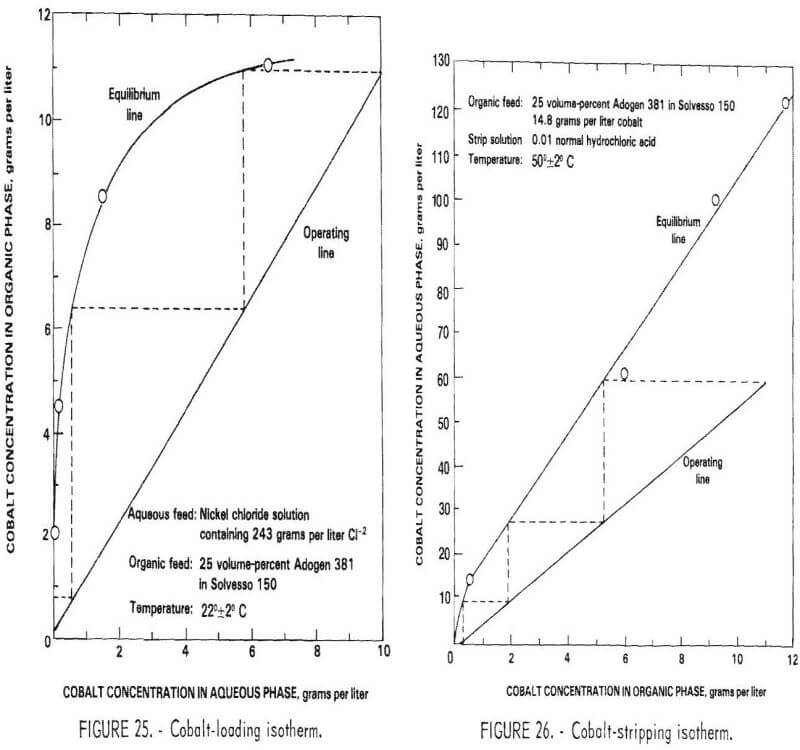
The initial tests were conducted using a synthetic raffinate containing 168 grams per liter of Ni, 10 grams per liter of Co, and 243 grams per liter of Cl-2. The solution was contacted at various phase ratios with a mixture of 25 volume-percent Andogen 381 (triisooctylamine) hydrochloride in Solvesso 150 at 22° C. The cobalt-loading isotherm constructed from these tests is shown in figure 25. As can be seen, three theoretical stages are necessary to reduce the concentration of cobalt from 10 grams per liter in the aqueous solution to the desired level of 10 milligrams per liter.
The loaded organic was stripped with 0.01-normal hydrochloric acid at various phase ratios to generate the cobalt-stripping isotherm shown in figure 26. Three theoretical stages were needed to produce a strip liquor containing 60 grams per liter of cobalt and a stripped organic containing approximately 0.2 grams per liter of cobalt.
As noted previously, difficulty was expected in separating the aqueous and organic phases in a mixer-settler system. The reciprocating-plate column has been developed to improve phase separation. A 4-centimeter-diameter column, similar to that described by Dim, having adjacent plates moving 180° out of phase was used for subsequent continuous extraction tests. The loaded organic phase was stripped continuously with 0.01-normal hydrochloric acid in two mixer-settlers.
The results of these tests are contained in table 22. Entrainment of the aqueous phase in the loaded organic was very low in these tests, producing a composite strip liquor with a cobalt-nickel ratio of 650. Note that the operating efficiency of the column was better at the higher throughput because it facilitated operation under more favorable values of plate amplitude and frequency. Considerably higher throughput with no loss of efficiency would be produced in a circuit specifically designed for the job.
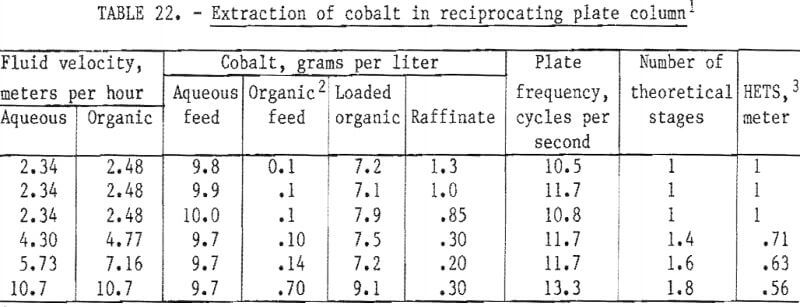
Discussion
The experimental program demonstrated the viability of a tertiary amine solvent extraction system using reciprocating plate column for extracting cobalt. The principal factors that affect this system are the efficiencies of the preceding raffinate purification steps, since iron and molybdenum are also extracted by the system chosen. The hydrolysis test results indicate that the levels of these elements should be low enough that major contamination of the cobalt circuit should not occur.
Copper is also extracted by the tertiary amine system, and some means of copper removal must be provided prior to electrowinning of cobalt. The raffinates from the continuous leaching tests contained an average 0.15 gram per liter of copper, but concentrations as high as 0.5 gram per liter were observed owing to flow surges and incomplete cementation. The copper content of the raffinate in a commercial facility should be more stable and average perhaps 0.1 grams per liter. This small quantity of copper could be extracted with one of several solid ion exchange resin systems. No experiments on copper removal were conducted in the present pro¬gram because the technology was considered well developed.
Removal of sulfate in a continuous operation would be done prior to solvent extraction by adding barium carbonate in a settling tank. The resulting barium sulfate would be discarded. No experiments on sulfate removal were conducted.
Electrowinning
A number of methods can be used to produce nickel and cobalt metals from the purified chloride liquors. However, electrowinning seemed to be a logical choice, since it was most compatible with the leaching circuit; that is, spent electrolyte, cathode rinse water, and chlorine generated in the electrolytic cell can be fully recycled. Also, the metal product is quite suitable as a melt charge material for superalloy production.
Experimental Work
Very limited work was done on electrowinning because it involves well-known technology which is widely employed on a commercial basis. In the case of cobalt, the quantity of electrolyte derived from superalloy matte was so limited that a meaningful demonstration could not be conducted. Considerably more nickel electrolyte was produced during the preceding hydrometallurgical work; consequently, it was possible to conduct a nickel-electrowinning test. The principal objective was to determine whether any trace element impurities derived from the scrap feed would interfere with nickel deposition or contaminate the product.
A bench-scale test was conducted on electrolyte derived from previous studies. The composition of the feed is shown in table 23. Titanium inert anodes coated with a platinum-group metal oxide were used. Kenekaron anode bags were employed. Cathodes were AISI 316 stainless steel masked with epoxy to produce 1-inch-diameter round buttons. The circuit was operated for 103 hours at a current density of 250 amperes per square meter at 70° C. Current efficiency was calculated to be 93 percent.
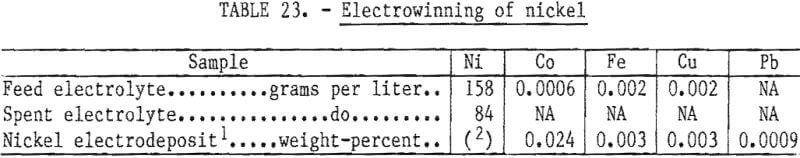
The appearance of the nickel deposit was very good, although a few pits were observed. The composition of the product is shown in table 23. The purity of the material compares favorably with that of commercial grades of electrolytic nickel, and the material would be acceptable for use in superalloys.
Discussion
The test described above was encouraging in that it demonstrates that good-quality nickel can be produced from superalloy scrap using the matte separation process. It should be emphasized, however, that the scrap material used in the melt stock was “vacuum grade” quality and thus represents the upper range of scrap metals purity that would be treated in a recovery process. Lower quality scrap feed will contain tramp elements which, if not removed at some point in the process, will cause difficulty in the electrowinning of both cobalt and nickel. An assessment of the individual or combined effects of trace elements is a difficult task which was well beyond the scope of this experimental program.
General Discussion
The laboratory-scale testing program has shown the technical feasibility of the process flowsheet initially selected for recovery of metals from complex superalloy scrap. The basic concept of separating chromium from the other metals by forming a matte in which chromium sulfide is a distinct phase was substantiated, although the separation efficiency was not as good as anticipated. Also, considerable difficulty in separating the sulfide phases by physical methods was experienced. Leaching of the metallic and nickel sulfide phases was quite effective. It was found that, if the redox potential was controlled, the chromium sulfide phase remained inert during the leach, and chromium remained in the leach residue. The principal uncertainty for the process that could not be tested fully was the range of variation of feed composition that could be accommodated by the process.
Material Balance
All of the test work discussed in this report was conducted on a batch or limited-unit continuous basis. It was not possible to test a fully integrated system of the type that would be employed in a commercial plant. To provide information on quantities and compositions of process streams at each stage of the flowsheet, as well as probable compositions and quantities of products, a material balance for an integrated system was prepared for the hypothetical “aim” composition. The compositions and element distributions at each stage of the recovery process are shown in table 24. The streams are identified by number on the table and on the simplified flowsheet shown in figure 27. Following solvent extraction (H), approximately 7½ percent of the nickel electrolyte is separated from the circuit. This is reacted with sodium carbonate to form a nickel carbonate residue and a sodium-rich effluent. The nickel carbonate is reverted to the hydrolysis stage to precipitate Fe and Mo impurities prior to solvent extraction. The nickel carbonate is thus used for maintaining purity in this part of the process. Note that with reverting of nickel electrolyte and nickel carbonate from solvent extraction back to the primary leach and hydrolysis stages, the quantity of solution and nickel at stages E, G, H, and I will increase almost twofold before stabilizing. Concurrently, the concentrations of Cr, Co, Mo, and Fe will decrease at stages E, G, and H. The final concentrations are approximated in table 24, key No. 8.
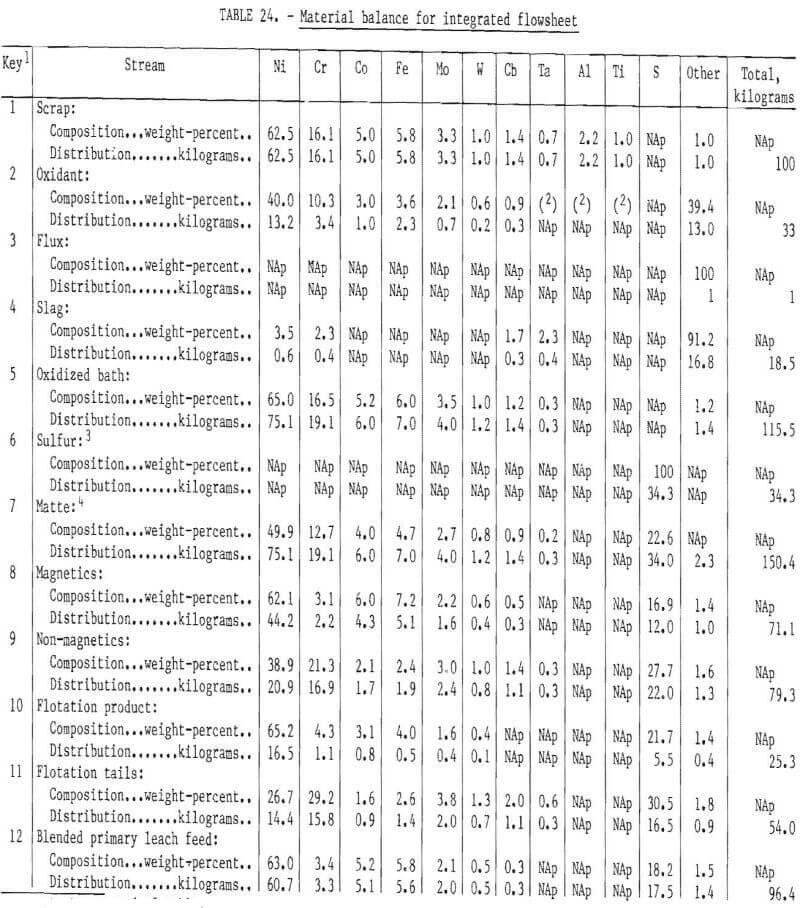
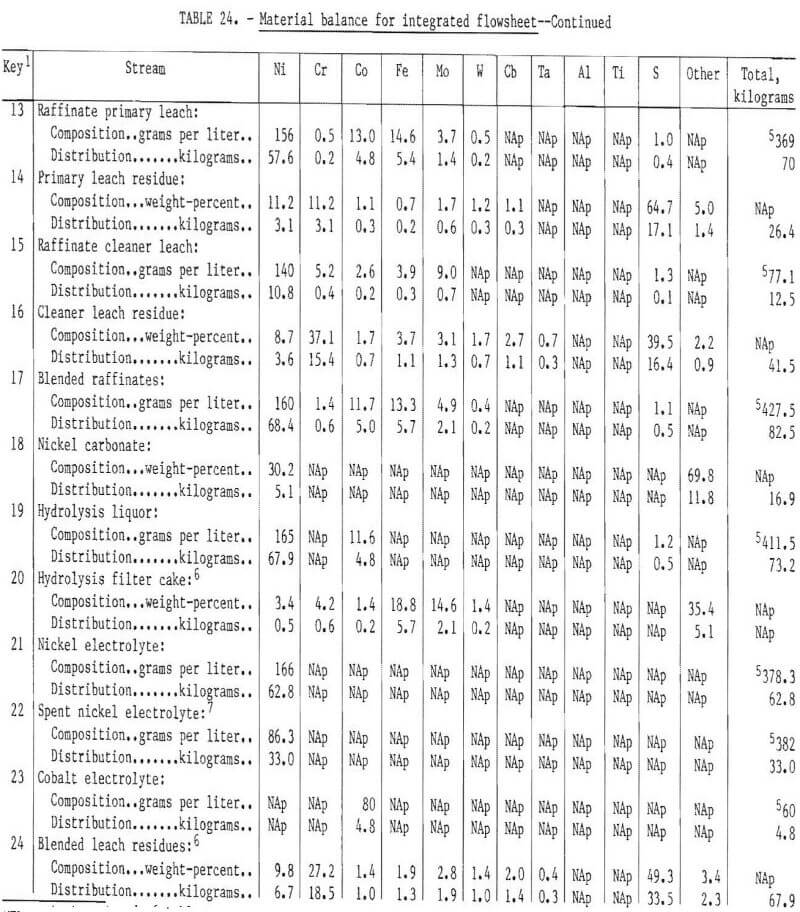
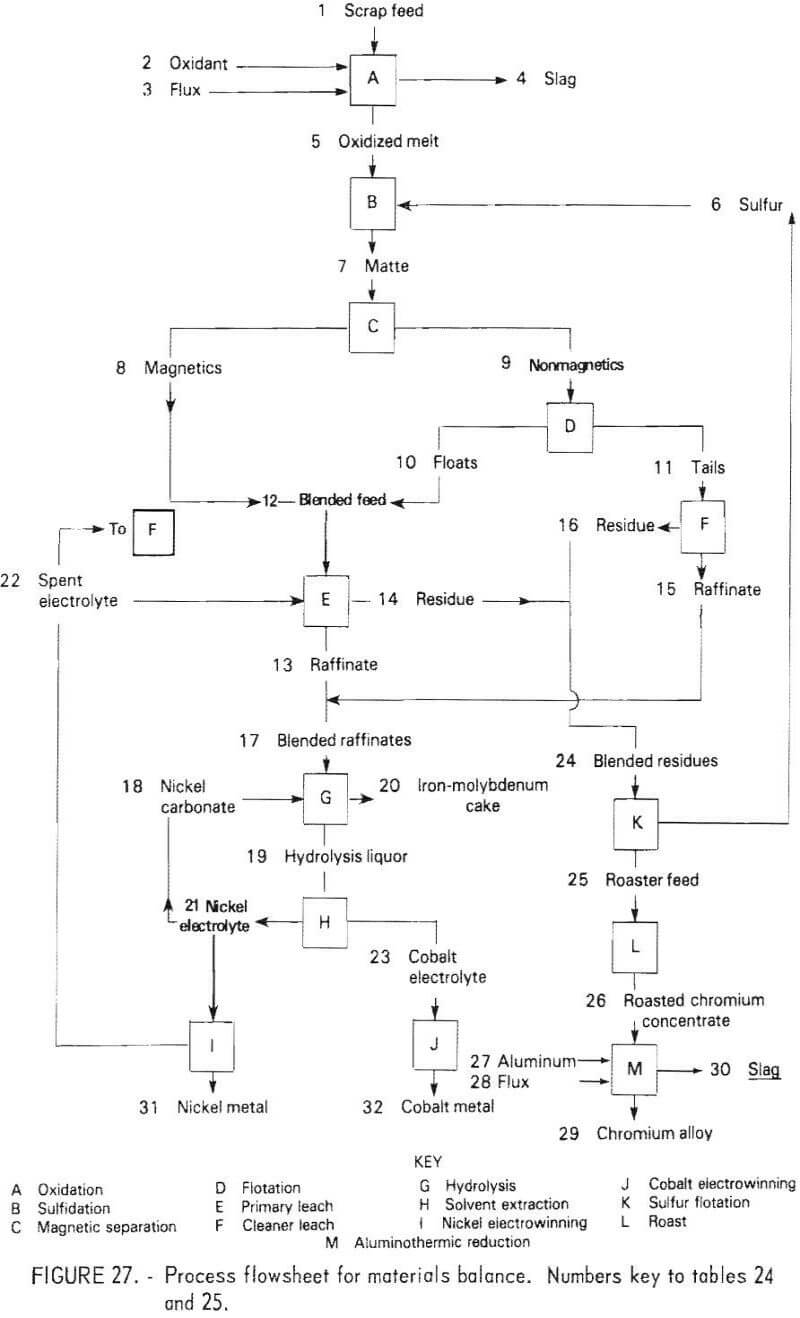
Obviously some variation in feedstock composition would occur for a commercial plant. Owing to the complexity of the process, it is difficult to predict the effect at the various intermediate stages of the process. The role of iron in altering the structure of the chromium-rich sulfide phases was discussed earlier, and an upper limit of 15 percent iron in the composite feed was suggested. There are probably limits for the concentration of most of the elements present in the feed beyond which the separation efficiency and quality of the products will decline. One objective of a pilot plant operation would be to identify these limits or to show that composition variations within the expected operating range do not cause difficulties.
Recovery of Metals
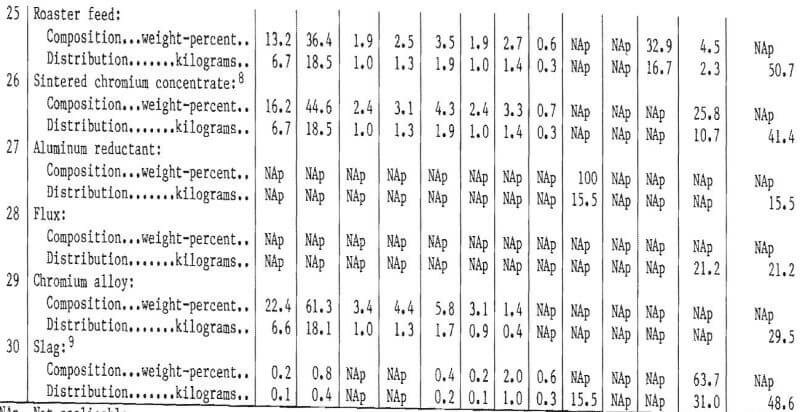
The material balance shown in table 24 provides the basis for predicting the recovery of metals in the overall process. It also provides a means for judging the efficiency of each step of the operation. The projected recovery of metals in the various products, byproducts, and wastes is shown in table 25. In this determination, as well as for the material balance, it was assumed that 100 kilograms of metallic scrap of “aim” composition was treated. The oxidant was 33 kilograms of fully oxidized scrap (flue dust, mill scale, or chemical process sludge) of the “aim” composition.
Thus the weight of the feed of 133 kilograms includes 17.2 kilograms of unrecovered material such as 0%, Al, and Ti. The three principal products are pure electrolytic Ni (90 percent of Ni in feed), pure electrolytic Co (80 percent of Co in feed), and Cr alloy ingot (93 percent of Cr, 9 percent of Ni, and 16 percent of Co in the feed). It is assumed that each of these products will be of a quality suitable for reuse by superalloy melters.
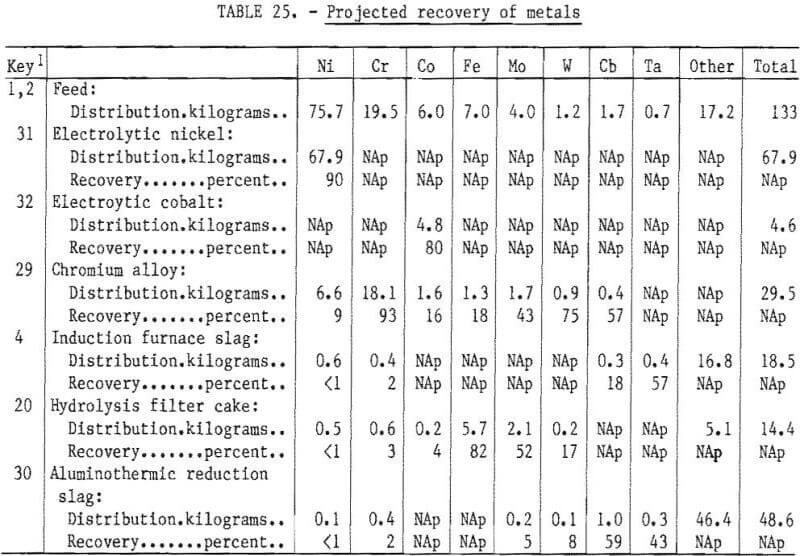
The fourth valuable product is the roasted hydrolysis filter cake, which will contain at least 50 percent of the molybdenum in the feed. This Fe-Mo oxide also contains some Ni and Cr. This cake could be processed to further concentrate the molybdenum, or it could be used directly as an additive for stainless or alloy steel production.
Recovery of metals in the four products in which their full value would be realized is Ni—99 percent, Cr—93 percent, Co—96 percent, and Mo plus W—92 percent. The losses of these metals are either in the slag or in a product where the full value would not be recovered, such as cobalt in the hydrolysis cake.
Columbium and tantalum are largely present in the induction furnace and aluminothermic reduction slags. The concentration of these metals in the slag will be on the same order as their concentration in the scrap feed. It may be possible to recover these elements through further processing; however, it is assumed that the byproduct value of these metals will be very low.
Improvement in Operating Efficiency
The material balance and predicted distribution and recovery of elements are based on results achieved in laboratory work. Clearly there are a number of points in the overall operation where conditions could be varied to increase or decrease the recovery of specific elements. For example, the recovery of Columbian and tantalum is influenced by the degree of oxidation of the molten metal bath. It may be possible to recover substantially all of these metals in the induction furnace slag at the expense of somewhat lower chromium recovery. Perhaps the most important variable is sulfur content of the matte. By lowering the sulfur content of the matte to about 18 percent, the Co, Fe, and Mo content of the chromium sulfide phase and the amount of free molybdenum sulfide are significantly reduced. The resulting Cr alloy would be more nearly a binary alloy of Cr and Ni, thus improving its acceptability, while the hydrolysis cake would be enriched in Mo. It is also known that a portion of the heavy metals in the leach residues are in the form of carbides and mixed heavy metal sulfides. It may be possible to remove these metals in a concentrate by floating the chromium sulfide after sulfur flotation. These points should be taken into consideration in any future studies to further develop the overall process.
Alternative Processing Methods
Although a few modifications were made, the viability of the basic flowsheet was demonstrated in the experimental program. One major modification that merits further consideration is the use of direct leaching of ground matte to separate nickel and chromium. This alternative procedure is based on the observation that dissolution of chromium sulfide can be suppressed by limiting the redox potential during leaching. The chromium-rich residue of such a direct leach process would be treated to produce chromium metal using fluidized-bed roasting and aluminothermic reduction. The major advantage would be elimination of the magnetic separation and froth flotation operations, which were found to be rather inefficient.
Conclusions
The technical viability of a novel process to recover chromium and other metals from superalloy scrap has been demonstrated. An experimental program using laboratory-scale equipment verified that the following processing steps were viable and capable of providing metal products suitable for reuse in superalloys,
- Mixed alloy scrap was melted and homogenized, after which a solid reducible oxide was added to oxidize the reactive metals (Al, Ti, Hf, Zr, etc,) present in the charge. The slag was skimmed and reserved for possible recovery of Cb and Ta.
- Sulfur was added to the bath to form a partial sulfide matte containing 17 to 25 percent sulfur. The matte was cast into an insulated mold for slow cooling to develop a coarse grain structure.
- The solid matte was crushed and ground to liberate particles of the three phases (chromium sulfide, nickel sulfide, and nickel-rich metal).
- Wet magnetic separation was applied to remove the magnetic portion of the aggregate. Nickel and chromium sulfides were separated using froth flotation. Owing to the friable nature of the sulfides and the difficulty in floating nickel sulfide fines, this portion of the flowsheet was found to be least efficient.
- The magnetic fraction was combined with the nickel sulfide flotation concentrate and then was given a hydrochloric acid-chlorine leach under controlled redox potential. This resulted in nearly complete extraction of Ni, Co, Mo, and Fe and rejection of chromium as a sulfide in the residue.
- The leachate was hydrolyzed by the addition of nickel carbonate to remove iron and molybdenum. In a commercial operation the resulting filter cake would be roasted to produce a granular oxide suitable for use in the stainless and alloy steel industries,
- A tertiary amine solvent extraction system was used to separate nickel and cobalt from the chloride leach liquor.
- Pure nickel metal was produced from the nickel-bearing solution by electrowinning.
- Cobalt metal would be electrowon from the cobalt-rich solution in a commercial plant. Insufficient material was available to demonstrate this operation on the laboratory scale.
- The chromium concentrate tails were also leached to remove any nickel sulfide not separated during flotation.
- The chromium sulfide concentrate was roasted to the oxide in a fluidized-bed roasting system. A chromium oxide product containing 0.02 percent sulfur was produced.
- The granular chromium oxide sinter was blended with aluminum powder and fluxes and reduced to a chromium alloy ingot. A fully metallic chromium- nickel ingot containing 0.033 percent sulfur was produced.
Based on laboratory work, the estimated recovery of the principal elements in a form that recovers their full value was Cr—93 percent, Ni—99 percent, Co—96 percent, and Mo—92 percent, Columbian and tantalum were contained in the melting furnace and aluminothermic reduction slags. Treatment of these slags to recover these elements may be possible.
An alternative scheme that could simplify the flowsheet was identified but not exhaustively investigated because of time limitations. This procedure is based on the observed inertness of chromium sulfide when the redox potential is carefully controlled during the leach. The feasibility of directly leaching a granulated matte was demonstrated on a bench-scale batch test. Further exploration of this promising approach appears justified.
