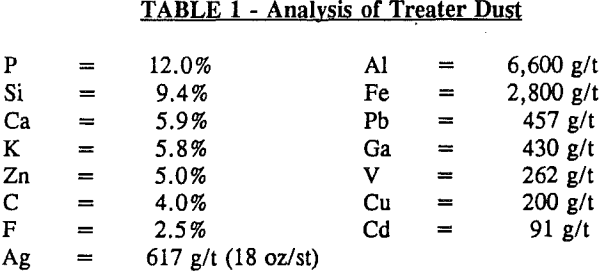
Treater Dust is the name given to the material that is collected in electrostatic precipitators used for off-gas cleaning of elemental phosphorus furnaces. Elemental phosphorus is made by reacting phosphate ore, silica, and coke in an electric furnace, as shown by these reactions:
- 2Ca3(PO4)2 + 6SiO2 → P4O10 + 6CaSi03
- P4O10 + 10C → P4 + 10CO
The treater dust contains the major elements of the electric furnace operation, such as phosphorus, silica, and carbon. In addition, minor elements in the phosphate ore, such as zinc, silver, and gallium, are concentrated in the dust through volatilization in the electric furnace.
Plant Overview
The process plant utilizes drying and grinding, two-stage sulfuric acid leaching, and cyanide leaching. The phosphorus, gallium, potassium, and zinc are solubilized in the acid leach operation. The silver is solubilized in the cyanide leach operation. Gallium is recovered by solvent extraction from the phosphoric acid, while silver is recovered from cyanide leach liquors using zinc dust cementation. Cyanide is recovered by a regeneration system using acidification and alkaline absorption.
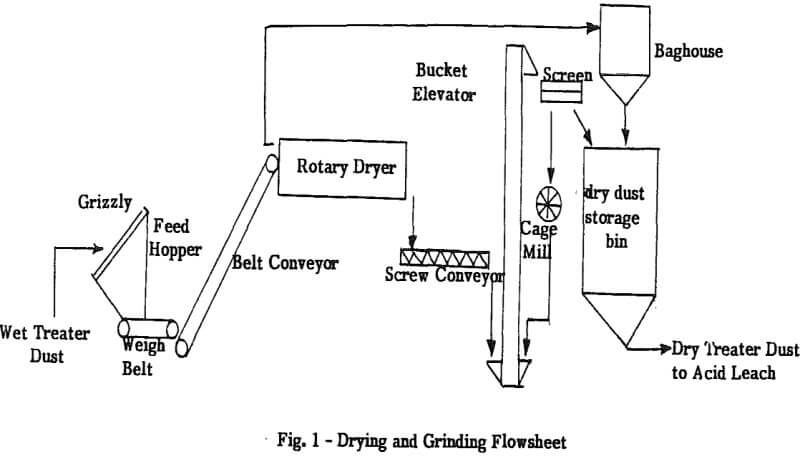
The oversize is generally composed of hard slag pieces and fused agglomerates. The agglomerates can be broken down by running over them with the front-end loader. The slag pieces are not easily crushed and are pushed to a discard pile.
Acid Leaching
The acid leach is the most important operation in the plant. High acid leach efficiency of the phosphorus and zinc is needed to provide high extraction of the silver in the cyanide leaching, with low cyanide consumption. Correct wash ratios in filtration are needed to maximize the phosphoric acid concentration, and minimize the shipping of water. Proper acid/treater dust ratios are needed to achieve high gallium extraction with a correspondingly high pH, thereby minimizing the ammonia used for neutralization.
A common feature of phosphoric acid plants is fluorine in the ore reacting with silica and sulfuric acid, generating gaseous silicon tetrafluoride. In the treater dust plant, silicon tetrafluoride is generated mainly in the acid digest tank, where high acidity and high temperatures predominate. Small amounts of silicon tetrafluoride are also generated in the two acid leach tanks; little silicon tetrafluoride is released in the preleach tanks. All leach tanks are covered and ducted to a single wet scrubber.
Gallium Solvent Extraction
Gallium is recovered from the phosphoric acid using solvent extraction. The solvent is composed of 20% Lix-26, 15% nonyl phenol, and 65% 470-B carrier. A total of 12 mixer/settlers are used – three for extraction; three for solvent washing; three for stripping; and two for acid scrubbing.
The phosphoric acid from the preleach operation is adjusted to a pH of 1.3-1.5 using NH3. This pH-adjusted solution is polish-filtered to remove any solids prior to solvent extraction.
Lix-26 was then tested and found to be effective in extracting gallium from treater dust solutions. However, it was found that hydroxyquinoline extractants, such as Kelex-100 or Lix-26, will not extract gallium from sulfuric acid solutions, although they do seem to be effective in phosphoric acid solutions. Table 4 shows the effect of phosphorus in an acid system on gallium extraction with Lix-26.
