Table of Contents
The objective of this study is to evaluate methods to improve the selectivity in flotation of the slightly soluble minerals, typified by calcite, fluorlte and scheelite. The separation of minerals within this class has long been one of the most difficult tasks facing the mineral processing engineer.
The slightly soluble mineral class, as defined herein, have solubility product constants, of very approximately, ksp 10 -10. Typical examples of minerals in the slightly soluble class include the following:

These may be concentrated from ores for their metal content or for use as an industrial mineral, but, just as importantly, they also commonly occur as accessory minerals in ore deposits. Either their recovery (i.e., flotation of trace amounts of malachite from a copper ore containing primarily sulfides) or their rejection (i.e., rejection of calcite during scheelite flotation from a skarn type of ore deposit) will affect the grade, recovery and profitability of an ore deposit.
Only a few separations of the slightly soluble minerals are made commercially (e.g., fluorite-calcite, bastnasite-barite, fluorite-barite). Usually, separation of minerals of this class meets with indifferent results; yet the minerals often occur together.
An important domestic resource beneficiation problem is the selective flotation of scheelite from the skarn type of deposit. Here the scheelite is associated with fluorite and much limestone and marble. Selectivity is necessary not only between scheelite and calcite but also between scheelite and fluorite. The inability to reject nearly all of the fluorite, say, would so dilute the WO3 grade of the concentrate as to make it unacceptable. As a consequence it must he treated by an additional hydrometallurgical step to make a saleable concentrate. In view of the importance of tungsten to any industrial economy, a selective separation here would be of significant benefit. A recent survey of the nation’s tungsten resources led to the following conclusion: “The United States has the potential for adequate tungsten production into the foreseeable future, provided it is willing to pay the price of essential research, extensive exploration and the processing of high-cost, low grade ores”.
There are other examples of major beneficiation problems of national importance with the slightly soluble minerals. For example, in the processing of phosphate ores, some of the Florida phosphate deposits are known to contain magnesite or dolomite, and these minerals are difficult to reject during the flotation of hydroxy-apatite. The phosphate industry is a major one, and in 1980 nearly 120 million tons of such ores were treated in the U.S.
The initial thrust of this study dealing with the slightly soluble minerals was with scheelite and those minerals closely associated with it (fluorite and calcite), though the longer range goal is to develop basic information to be used to improve the separation of any mineral in this class from any other mineral either within or outside of this class.
Separation of the slightly soluble minerals (Ksp 10 -10) one from the other is one of the more difficult tasks confronting flotation engineers. This study has focused on means of separating such prominent members of this class as scheelite, fluorite and calcite from one another. Some experimental work has also been done on the minerals magnesite, dolomite and apatite.
Carboxylic acid-type collectors are commonly used industrially to float all of these minerals. Thus, the problem at hand is to ascertain means of achieving selectivity between minerals known to respond rather similarly in flotation. Four techniques have been proposed for the separation of minerals of this class from each other:
- Control of potential determining ions
- Use of stereo-specific collectors
- Use of flotation rate differences
- Use of selective activating and depressing compounds.
This study was directed toward a cursory evaluation of each of these techniques, and each was found to be a possible method of achieving selectivity. Stereo-specific collectors — those whose parking area may closely match the available adsorption site on one mineral but not on another — are a possible means of achieving selectivity between scheelite, fluorite and calcite. Stereo-hindered carboxylic acid salts and sulfonates all show selectivity between the three minerals tested. Separations also appear to be possible by the controlled use of the lattice ions (e.g., Ca++ and WO4= for scheelite, CaWO4), H+, OH-, CO3= and HCO3- all of them ostensibly potential determining in the systems studied.
Flotation rate differences of the minerals as a function of pH under conditions of starvation collector usage also showed promise. Scheelite and fluorite float most rapidly with sodium oleate in slightly acidic (pH 4-7) solutions whereas calcite floats most rapidly near pH 10.
Use of Mn++, Pb++, or other heavy metal hydroxy complexes as activators was seen to effect each of the slightly soluble minerals differently. This is in sharp contrast to the oxygen containing, relatively insoluble minerals, where all minerals respond in a similar manner to such activation procedures.
Results indicate that all four methods outlined above may potentially be used to achieve flotation separation of the slightly soluble minerals. More extensive research should be undertaken to detail the specific applicability of each of the procedures and to make a more complete evaluation of the effect of collector type and concentration, mineral type, pH and size effects.
Possible Methods or Achieving Selective Separation
Because minerals of this class behave so similarly, their selective separation is a difficult task. It is further complicated since they are nearly all floated commercially with fatty acid collectors. Selectivity from other minerals in this class is thus difficult since the driving force for collector adsorption is the relatively low solubility of alkaline earth and other metal soaps in solution (Ksp 10 -15). This collector often works industrially because the mineral being floated is not accompanied by other minerals of the slightly soluble mineral class.
The literature is voluminous (see, for example, the excellent summary by Hanna and Somasundaran), but the development of selective flotation techniques for minerals of the slightly soluble class, one from another, has often eluded practical industrial application. Obviously, to accomplish such a separation will require procedures somewhat different than those used previously. Reliance will have to be made on subtle differences in the minerals, the reagents used and the interaction of these reagents with the mineral surface.
Possible methods for achieving selectivity include:
- Control of the potential determining ions followed by selective flotation with long chain amines, sulfate or sulfonates.
- The use of stereo-specific collectors; those whose parking area closely matches the mineral surface of one mineral but not another.
- Flotation rate differences.
- Selective depression.
- Selective activation.
Control of potential determining ions. Of first importance is the determination of the potential determining ions for minerals of this class. Twenty years ago Aplan and Fuerstenau concluded that the potential determining ions for this group of minerals were the lattice ions. In reality, for many of the slightly soluble minerals, the situation is found to be somewhat more complex, because through such equilibria as:
CO2(g) ↔ H2CO3(aq) ↔HCO3- ↔ CO3=
the ions HCO3-, CO3=, H+ and OH- may also be potential determining for the carbonate minerals. Through surface carbonation, calcium-bearing minerals under the appropriate conditions, may take on a surface coating of carbonate and respond as would calcite. Other listings of the potential determining ions for minerals of this class are given by Parks, Ney and, more recently, by Hanna and Somasundaran and Aplan. The review by Hanna and Somasundaran is particularly extensive.
Research in progress in our laboratories by Spearin, Simkovich and Aplan indicates that the potential determining ions for fluorite, CaF2, depends acutely on the history of the mineral and natural and induced point defects are of great importance. A knowledge of the properties of this mineral as it occurs in nature may be used to facilitate its selective flotation. For example, we have been able to separate white fluorite from the purple variety.
The use of stereo-specific collectors. Work by Parekh and Aplan used the critical surface tension of wetting method to study the adsorption of fatty acid collectors onto 10 minerals of this types. This study gave good evidence that selection of a collector with a fixed parking area may be used to affect a separation between various minerals of this class. This follows the earlier ideas of Schulman and Smith.
Flotation rate differences. Studies in our laboratory extending over a decade, but never pursued to completion, indicate that flotation rate differences may be helpful in separating various minerals one from another. This separation is not unlike that of the rejection of pyritic sulfur from coal during flotation as advocated by Aplan and his associates where the separation is achieved by taking advantage of differences in their flotation rates.
Selective depression. Depression may be accomplished by a wide variety of methods such as control of the potential determining ions, adsorption of an ion that will compete with the collector for the surface, sequestering an ion that would otherwise activate the mineral, surface oxidation, removal of a collector, resurfacing the mineral with a reagent (e.g., silicate, quebracho, starch) that will prevent collector attachment, etc., etc. Finding a depressant selective for a specific mineral is often complicated because depressants often tend to be somewhat non selective in their depressing action; particularly for those minerals which are mineralogically similar to the mineral in question. For example, quebracho is rarely used to depress calcite during scheelite flotation because, even in carefully controlled additions, it tends to depress some of the scheelite as well.
Selective activation. Activators and especially those of the metal ion hydroxy class tend to be non selective in their adsorption onto minerals. If such a process is to work, it will, of necessity, likely be based on subtle differences. One such possibility is to make use of self activation. Examples of this are the use of SO2 to “activate” manganese dioxide minerals with Mn++ and the self activation of chromite. Another possibility would be to make use of starvation quantities of the activating ion to facilitate selective adsorption onto certain minerals only.
Obviously it is not possible to investigate, in depth, all of these variables within the tine frame of the contract (1 year) and manpower available (1 graduate student). To cover the greatest amount of material efficiently, the various methods were surveyed and the number of minerals involved was limited.
Experimental Material and Methods
Minerals
The minerals used were apatite, calcite, dolomite, fluorite (both purple and white), magnesite and scheelite. Greatest emphasis was placed on scheelite, fluorite and calcite. All minerals were obtained from Ward’s Natural Science Establishment, Rochester, New York, except the Bishop, Emerson and Borralha scheelite which were obtained from Union Carbide Corp. and the British Columbia scheelite which was obtained as a gravity concentrate from Dr. George Poling, University of British Columbia. The latter material was further upgraded by sizing, magnetic separation and hand vanning. All scheelite flotation studies were made with this latter concentrate. For some of the Zeta Meter studies, synthetic scheelite, laboratory grade calcium tungstate was used. Since this material was found to contain chloride ion, which led to erratic results, it was washed with water and sedimented by centrifugation several times.
Chemicals
Standard laboratory chemicals were of analytical quality. Specialty organic chemicals were obtained from Eastern Kodak Co. and from Aldrich Chemical Co. Laurie acid (99%) and dodecyl amine (99%) were obtained from Armak Chemical Co. and the sulfonates were obtained from Shell and from Pilot Chemical Co. All water used was double distilled to insure purity.
Zeta Meter
A Riddick Zeta Meter was used for determination of the zeta potential of the minerals. Most minerals were first dry ground to -65 mesh and then wet ground for two hours in a Fisher automatic agate or porcelain mortar and pestle at pH 5.0-5.3 (this pH was used to minimize the possibility of surface carbonation). The ground slurry was then placed in 30 ml of water and allowed to stand to facilitate sedimentation of the coarser fraction. A few drops of the supernatent liquid, containing some of the finely ground mineral particles, were then transferred to 50 ml of solution of pre-set pH and ion composition and allowed to equilibrate for 3 hours prior to measurement.
Flotation
Flotation testing was done by using freshly ground, 100 x 200 mesh minerals and the Hallimond tube. The procedure used was that of Fuerstenau and Metzger except an extra coarse, glass frit replaced the capillary used for gas admission to the flotation cell.
Experimental Results
Potential Determining Ions
The Zeta Meter was used to evaluate the zeta potential of the various minerals in the presence of their lattice ions and of H+ and OH-; all thought to be potential determining ions.
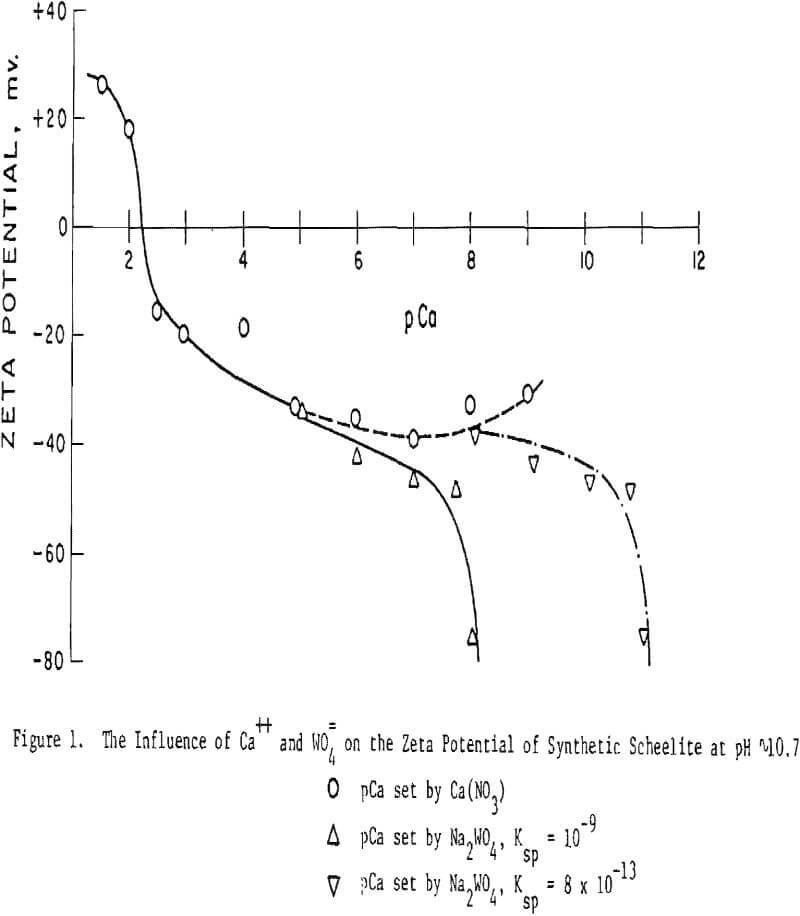
Scheelite. Figure 1 shows the influence of Ca++ and WO4= ions on the zeta potential of scheelite. Note that the abscissa is given in pCa units, defined similarly to pH, i.e., pCa = -log[Ca++] . It will be seen that the scheelite is negatively charged in all except rather concentrated Ca++ solutions, ∼ 10-² moles/liter and greater (pCa 2.2 = 6×10-³ M/L).
The pCa values for all curves listed in Figure 1 except those where the use of WO4= is indicated, were set by use of controlled amounts of calcium nitrate. For pCa values greater than ∼ 5 this procedure would not be strictly accurate because of the solubility product relationship of scheelite:
CaWO4 ↔ Ca++ + WO4=
ksp = 8×10 -13 (Latimer (21))
ksp = ∼ 10 -9 (Arnold and Warren (22))
However, because the number of particles and quantity of original supernatent liquid used from the grinding step was very small in our experimental method, we calculate that the influence of dissolved ions would have no effect above pCa ∼ 7. Its effect at higher pCa values would depend on the accuracy of the ksp values and rates of reaction.
When WO4= ions were used to set the higher pCa values, the curve shows a reasonable continuum using Ksp = 10 -9. This fact, plus mineral solubility studies, make us believe the value of 10 -9 is the more nearly correct. At pH 5 and 8, that portion of the curve set by use of WO4= does not ‘tie’ well into that set by Ca++. This is not unreasonable since tungstate ions exist only in alkaline solution.
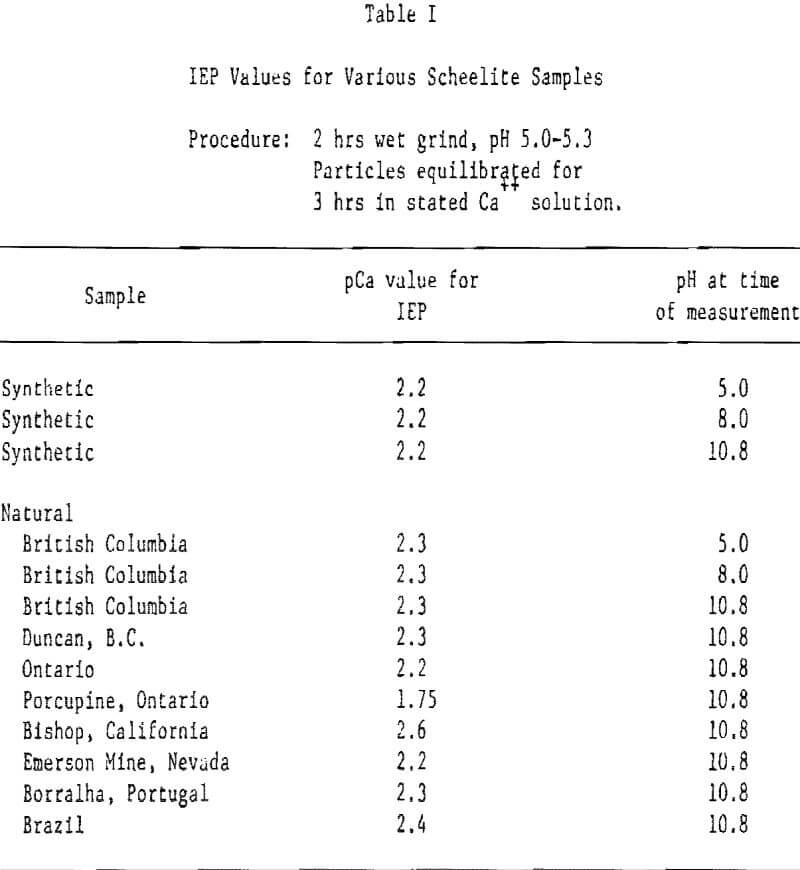
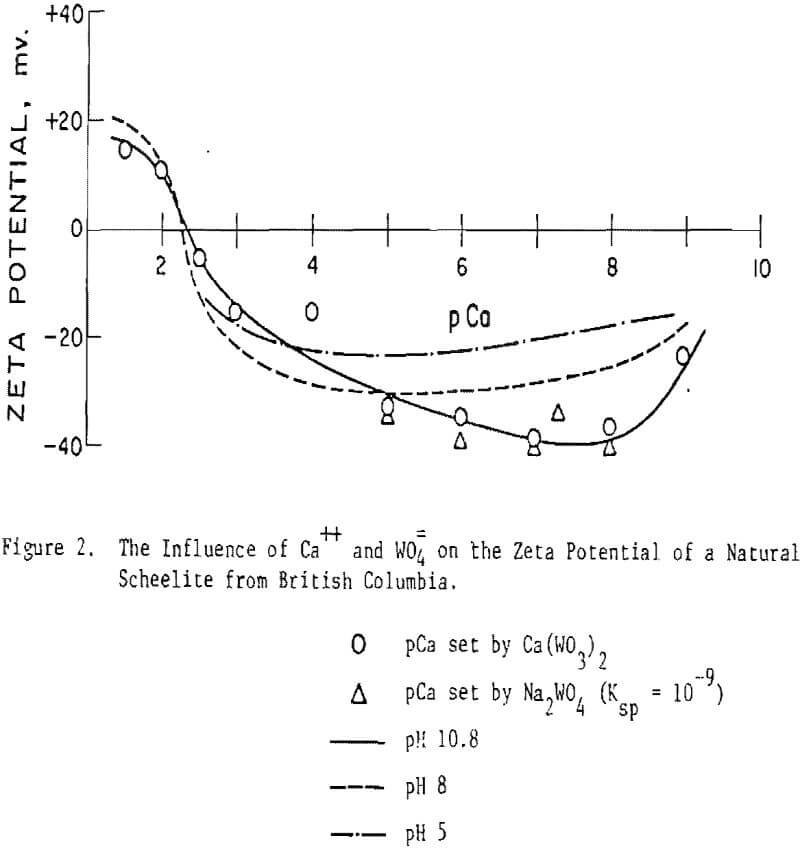
Results of additional zeta potential studies on synthetic and natural scheelite are given in Table 1 and Figure 2. Note that the isoelectric point (IEP) of scheelite occurs at about pCa 2.3 irrespective of the scheelite source or pH of measurement within the range pH 5.0-10.8. Apparently the surface carbonation noted for fluorite is not of sufficient magnitude in this case to alter the IEP since it remained constant over a broad range of pH values. Alternatively, it may be postulated that surface carbonation can occur even at pH 5 where but little CO3= and HCO3- are available to form the surface carbonate.
Figure 2 shows the curve obtained using a natural scheelite sample from British Columbia. The IEP occurs at pH 2.3 and is not affected by the pH of measurement over the range pH 5-10.8. Note that at pH 10.8 the data points, where the pCa was calculated from ksp = 10 -9 and a known concentration of sodium tungstate, agree well with those where calcium nitrate was used to set the pCa. In both Figures 1 and 2 the data point at pCa 4 lies above the curve as drawn. This response is found with most of the scheelite samples tested and is, thus, believed to be real even though artistic license has been used to draw in a smoothed curve. It is believed the location of this data point is due to the quantity and/or rate of calcium dissolution from the surface. Of course, pCa values greater than ∼ 5 to 7 may be subject to some doubt since an actual measurement of the Ca++ content of solution was not made but was set externally and/or based on the solubility product relationship.
Since scheelite is negative in all but rather strong calcium solutions, it appears that Ca++ dissolves from the surface much more readily than does WO4, and this prior assertion by Arnold and Warren can be generalized to a broad variety of scheelite samples and conditions.
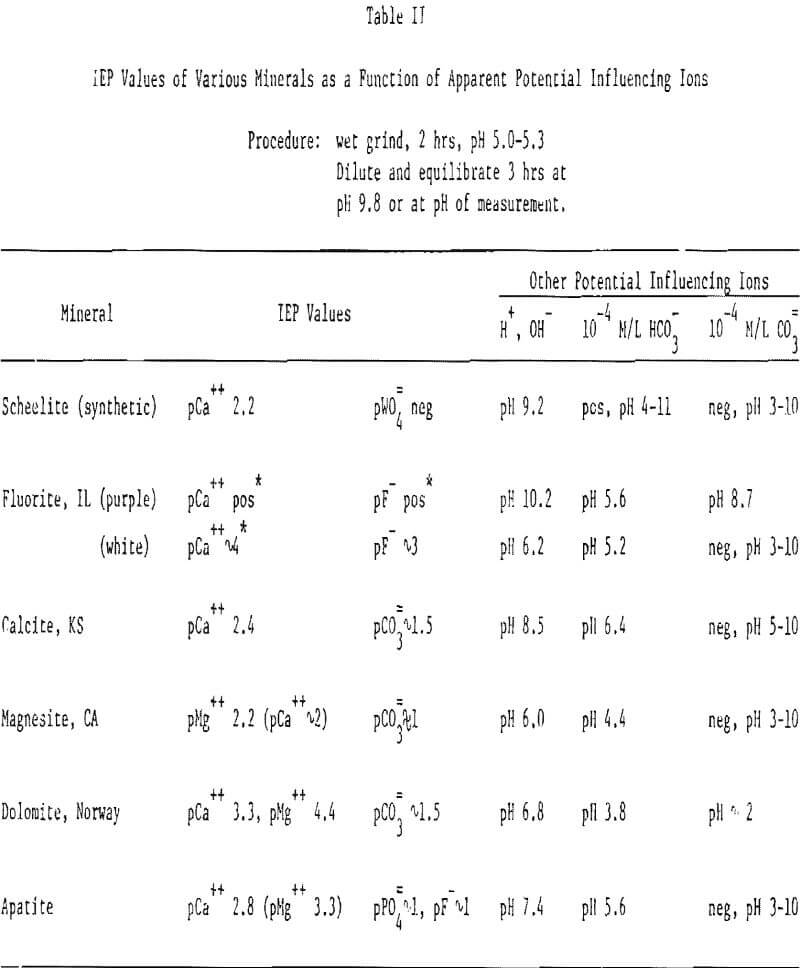
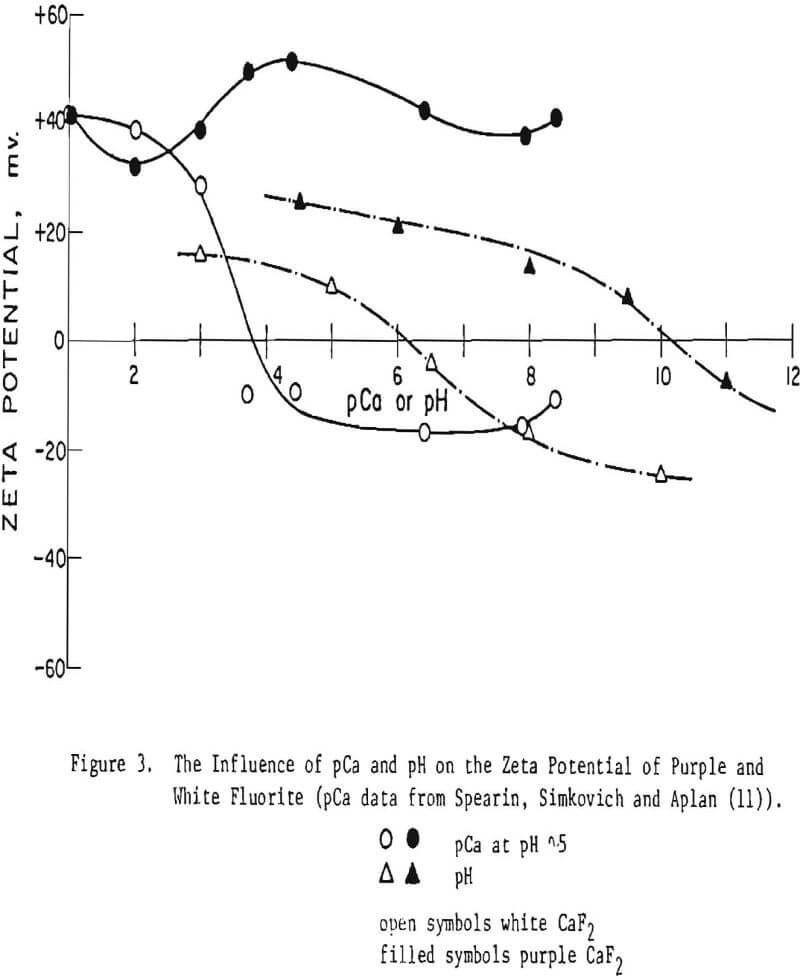
Fluorite. The zeta potential curves from white and purple fluorite are shown in Figure 3 and the data are summarized in Table II. Purple and white fluorite are seen to act quite differently than did scheelite and from each other. Purple fluorite has an IEP at pH 10.2 whereas white fluorite has its IEP at pH 6.2. The zeta potential of purple fluorite is seen to be always positive even in a 10-¹ M/L NaF solution (pCa 8.4, based on Ksp = 3.76 x 10 -11) whereas white fluorite has an IEP at pCa ∼ 4. Spearin, Simkovich and Aplan have previously reported on the difference in the electrophoretic response of various fluorite samples, and they have shown this is related to color centers and the point defect nature of fluorite. Recent findings in our laboratories have shown that the electrophoretic properties of this mineral are especially sensitive to the mode of preparation and are probably due to: (1) selective dissolution of the lattice ions, thus influencing the charge on the surface (2) the role of HCO3-. Our preliminary experiments indicate that the dissolution of Ca++ and F- from fluorite depends on the pH, the nature of the fluorite and the presence of HCO3-.
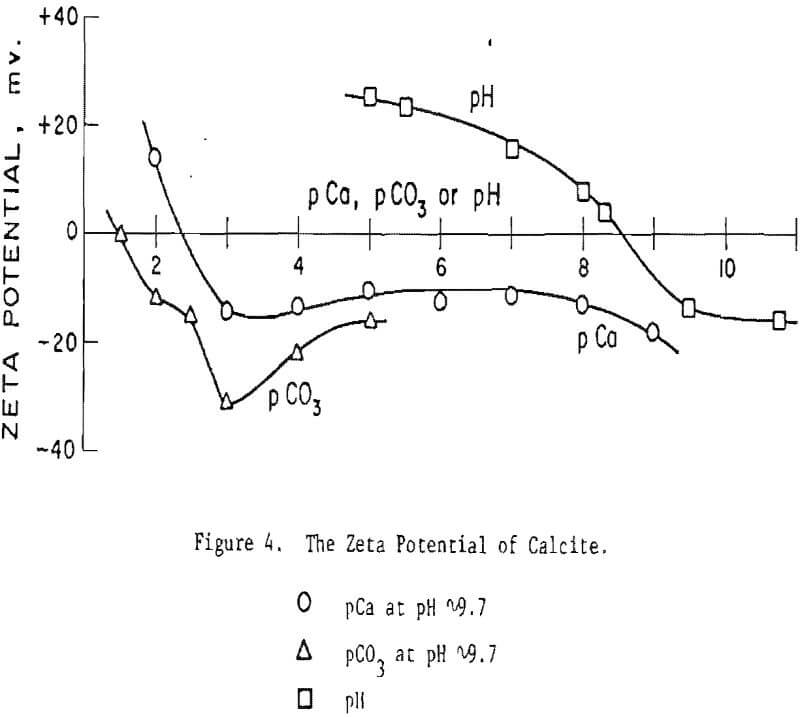
Calcite. Figure 4 indicates that Ca can reverse the sign of the charge of calcite only in strong solutions (>4 x 10-³ M/L Ca++). Note that Ca++ and CO3 appear to influence the calcite independently, especially in strong solutions of these ions. This gives strong support to the contention that selective dissolution and/or other competing reactions, occurring at the mineral surface, influence the charge on calcite. The apparent IEP at pCO3 1 may well be not real, but, instead, may represent the compression of the electrical double layer.
Other Minerals. The IEP data for several minerals are summarized in Table II. The initial grinding was done wet, at pH ∼ 5, to minimize surface carbonation and the samples ‘equilibrated’ prior to measurement as previously described. The data of Table II indicates just how complicated an understanding of the slightly soluble minerals can be. For most of the minerals studied (except purple fluorite), the lattice cation is potential determining but usually only in strong solutions (> ∼ 10-³ M/L). These data infer that the lattice cation dissolves easily, thus leaving the surface negative. In the case of scheelite and white (but not purple) fluorite, the anion appears to be potential determining. The lattice anions of the other minerals are either not potential determining or were prevented from acting so by other reactions or the experimental procedure used. The IEP values at ∼ 10-¹ M/L are likely an artifact due to the collapse of the electrical double layer resulting in a near zero zeta potential; the most probable way an anion might cause a change in charge from (-) to (+) (ignoring complexing reactions).
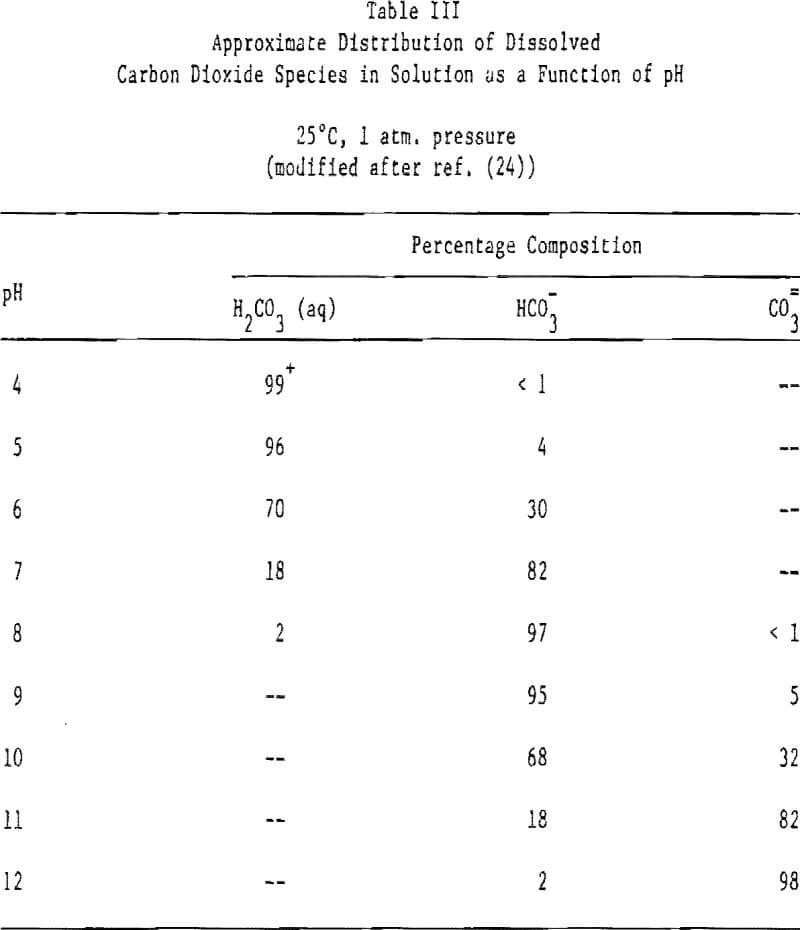
Hydroxyl and hydronium ions are observed to exert a charge controlling effect in all cases. The influence of the carbonate species, while pronounced, is more difficult to understand. Why the minerals should act differently in HCO3- and CO3= solutions, given the relationship between then at equilibrium as determined by pH (see Table III), and their reported fast rate of equilibration, is difficult to understand. This aspect is in need of much study.
Stereo-specific Collectors
The concept postulated here is that for certain mineral-collector systems there may be a better ‘size fit’ in forming a collector layer on the mineral surface with some systems than with others. The use of stereo-hinderance to affect specific attachment of a collector should be most pronounced in those systems where a specific site for collector attachment occurs. For the fluorite-oleate and similar systems, Peck and Wadsworth have shown the formation of calcium oleate on the surface. This implies, though does not prove, that oleate attaches to the calcium site. Using the critical surface tension of wetting technique, Parekh and Aplan have demonstrated that the cation adsorption site in the slightly soluble minerals can control the nature of the adsorbed collector.
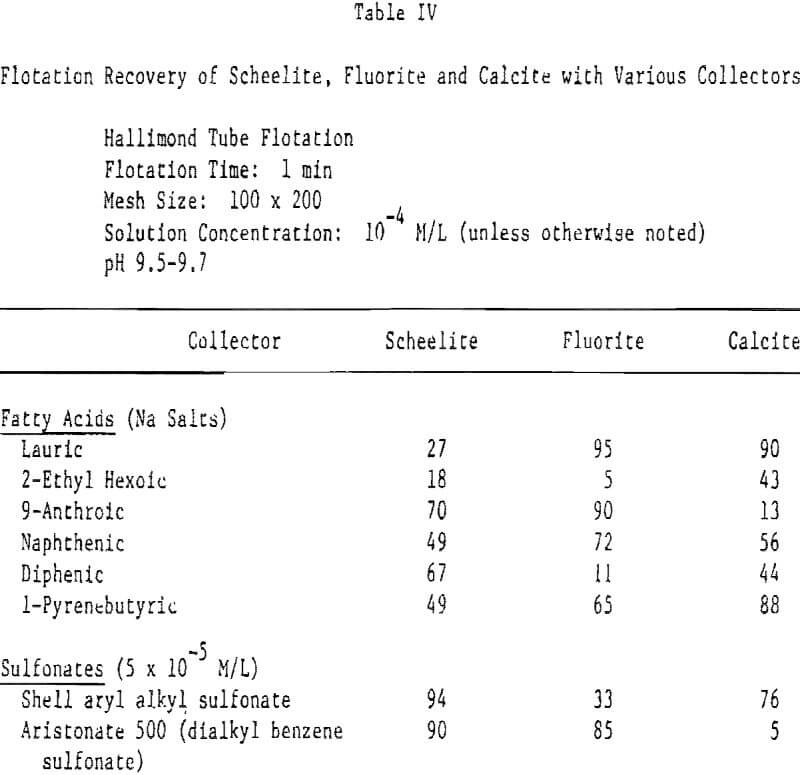
To test this premise, a series of fatty acids and sulfonates all with mono-ionic functional groups, but with widely differing hydrocarbon groups (and, hence, widely differing parking areas per molecule), were selected for testing. In a ‘scoping’ test these compounds were tested at a fixed pH (9.5-9.7) and fixed concentration (typically 10 -4 M/L). Table IV shows there is considerable merit in the basic premise that use of stereo-hindered collectors can be used to effect separations between the three minerals. Note, in nearly every case, the minerals respond quite differently to the various collectors. For example, diphenic acid floats scheelite well, calcite less so and fluorite poorly, whereas at this concentration lauric acid floats fluorite and calcite easily but not scheelite. It will be observed that flotation differences between these three minerals can be seen not only with the chemisorbed carboxylate but also with the less strongly adsorbed sulfonate collectors.
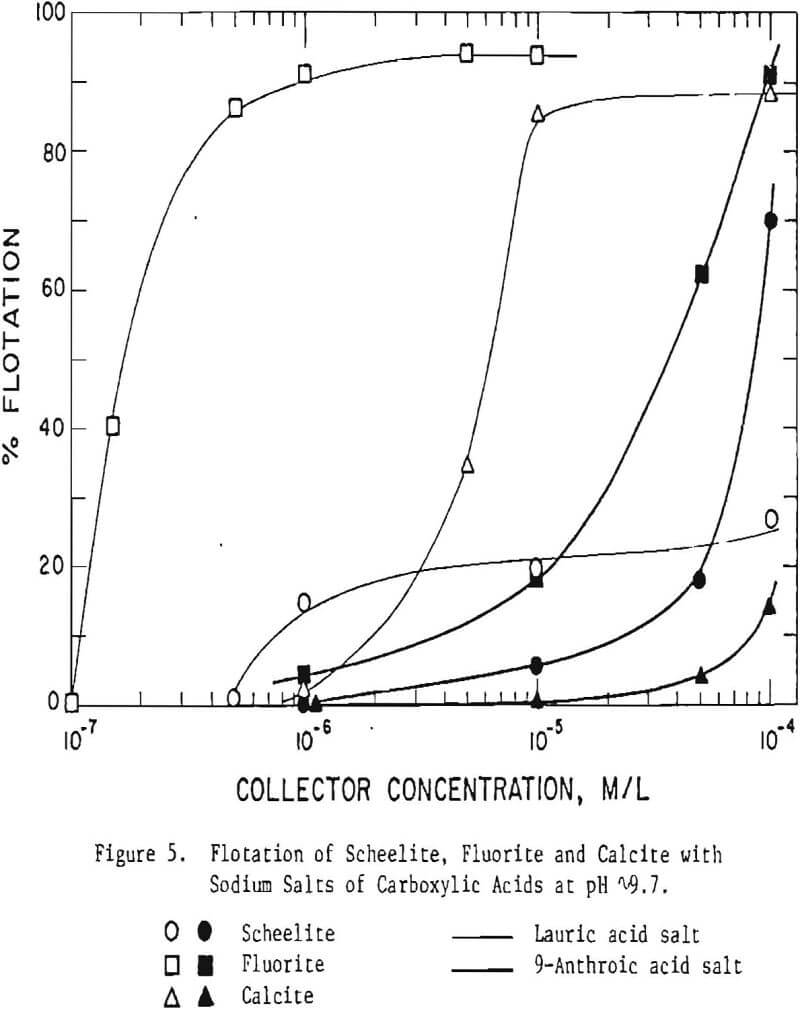
Figure 5 shows the effect of reagent concentration of two carboxylic acid collectors, the sodium salts of lauric and 9-anthroic acid, on the flotation of scheelite, fluorite (purple) and calcite. Based on these data a scheme can be postulated where starvation quantities of lauric acid would be used to separate fluorite from scheelite and calcite, and, subsequently, 9-anthroic acid would be used to separate scheelite from calcite.
Clearly stereo-hindered collectors show a preference for one type of slightly soluble mineral over another. A more thorough study of the effect of collector concentration, pH, particle size and mineral type must be conducted before application to separations in an industrial flotation plant can be considered.
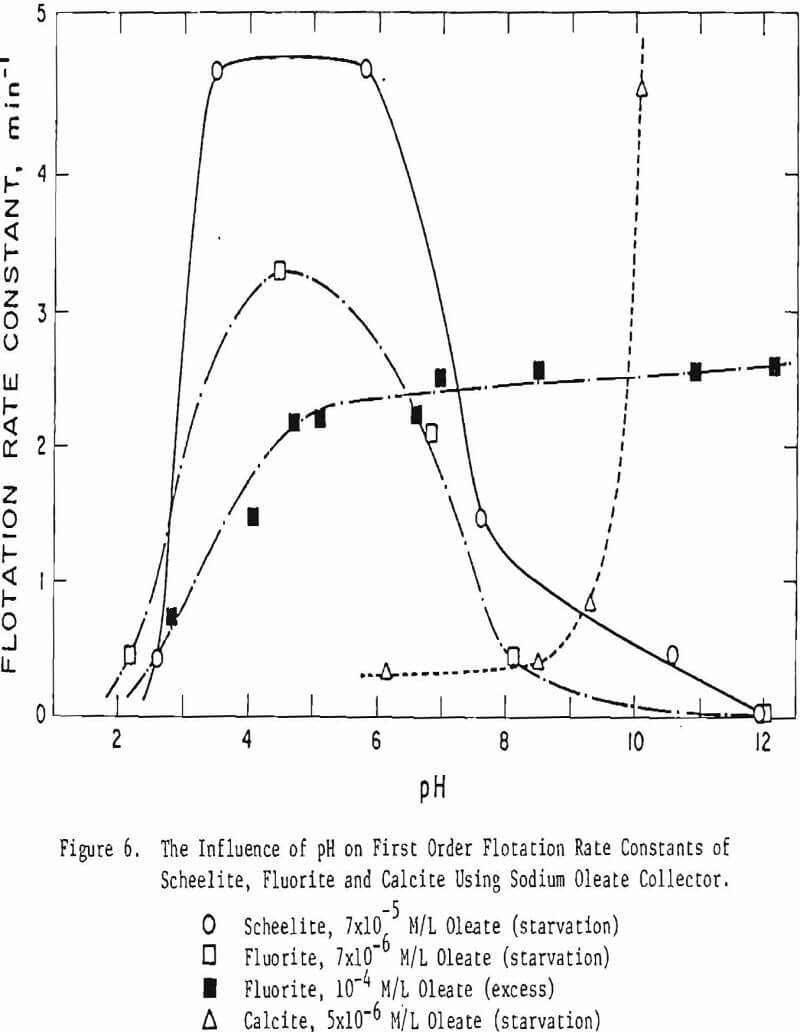
Flotation Rate Differences
Flotation rates are known to differ depending on the mineral and on the collector. Preliminary studies were made on the use of flotation rate differences to effect a separation of scheelite, fluorite and calcite. Using starvation quantities of reagents (at, or slightly above, the incipient sodium oleate collector concentration required for nearly complete flotation) mineral flotation rates were determined as a function of pH. By plotting time in minutes vs. the log cumulative percent remaining in the cell, the first order flotation rate constants may be determined from the initial straight-line portion of the curve:
k(min-¹) = 2.303 Log% Rem. @ t1 – Log% Rem. @ t2/t2 – t1
These data for the first order rate constants are then plotted on Figure 6. Note that fluorite and scheelite are best floated in acidic media (pH 5), though fluorite is floated with an order of magnitude less collector (7 x 10 -6 M/L for fluorite vs. 7 x 10 -5 M/L for scheelite). Increasing the amount of oleate to a much higher level (10 -4 M/L) reduced the flotation rate of fluorite near and below the pKa of the fatty acid (pH 4.7) but increased it in the alkaline region. Calcite, on the other hand, is best floated with a starvation collector concentration at pH ∼ 10. Obviously flotation rate can be used to affect a separation between these minerals (especially for the rejection of calcite), though additional studies to evaluate the influence of particle size are in order.
Activation and Depression
It was initially felt that control of the potential determining lattice ions could be used to separate one of the slightly soluble minerals from another; most especially the calcium containing ones: apatite, calcite, dolomite, fluorite and scheelite. Based on the data of Figures 1-4 and Table II, only fluorite and dolomite show any significant difference in their IEP values from the average at pCa ∼ 2.3. The anion appears to effect mainly fluorite, and perhaps scheelite. Control of pH in the region pH 6-10 offers some hope of effecting a separation of these minerals with colloidal electrolyte collectors since the IEP values range from pH 6.0 to 10.2. Control of the HCO3- and CO3= concentrations appears to be a more promising way of selectively regulating the surface charge on the minerals though why these two ions act differently, when they are reported to equilibriate rapidly, is not known.
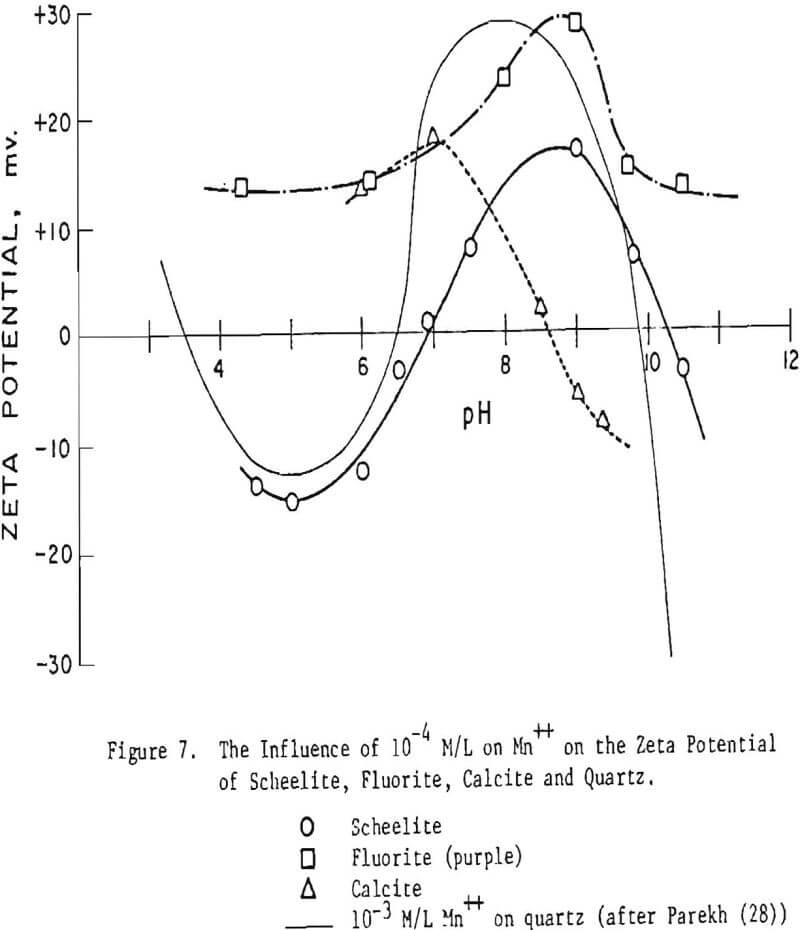
Another means of activation is through use of an external metal ion activator. James and Healy and Parekh and Aplan have indicated that oxygen containing minerals respond similarly to a given metal ion hydroxy complex. Figure 7 shows that the slightly soluble minerals respond differently to Mn++ than does quartz and differently from each other. Based on charge considerations, it should be possible, for example, to separate scheelite and quartz from the others at pH < 6.5 and fluorite from the others at pH > 10.5. Lead ion, data not shown, was also observed to effect the zeta potential differently for each of the slightly soluble minerals evaluated. Time did not permit this aspect to be explored further nor to delineate the effect of depressants which function by a resurfacing mechanism.
Conclusions
The test work reported here gives strong indication that a selective separation of one slightly soluble mineral from another should be possible through such methods as: (1) control of the potential determining ions, (2) stereo-specific collectors, (3) flotation rate differences and (4) selective activation and depression. These experiments have indicated that the; use of stereo-specific collectors should be especially fruitful, though in each of the other cases, substantial evidence indicating good potential for a selective flotation separations was also obtained. It is recommended that a much more comprehensive program be undertaken to evaluate these promising leads.