Table of Contents
Bureau of Mines forecasts of the aluminum situation show that U.S. demand for the metal in the 1960’s increased at a average annual rate of 9.4 percent, with a projected long-term growth rate of 6.5 percent, higher than any other metal. Conversely, the Nation is becoming increasingly dependent on imports for its aluminum needs. Of the U.S. demand for primary aluminum in 1972 (5,083,000 short tons), only 9.2 percent was supplied by domestic sources. Furthermore, almost 800,000 tons of aluminum metal was imported, or 100,000 tons more than in 1971. The value in 1972 of these imports was
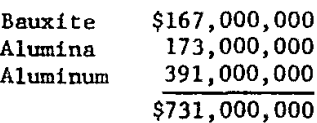
If only aluminum metal had been imported, the import value, at 25 cents per pound, would have been $2.6 billion. The exporting nations realize that this increase in value exists and that their dollar incomes increase in proportion as higher value products are exported. Carried to the extreme of importing only aluminum metal, the annual bill for our balance of trade in 30 years could be $25 billion.
To complicate the situation, the competition with other industrial nations for aluminum resources is increasing. Eventually the situation may become analogous to the current one involving the world’s petroleum output.
From the standpoint of national interest, there is an increasingly urgent need to advance a technology that will allow the United States to depend on its own ample resources .for aluminum production. For the long term, our reserves of bauxite are essentially negligible. On the other hand, Bureau of Mines Information Circular 8335, entitled “Potential Sources of Aluminum” and published in 1967, reported that there were over 160 billion tons of alumina in the United States in sizeable deposits of anorthosite, clay, laterites, shale and low-grade bauxites. The 1967 report did not cover other resources that could well become important to domestic alumina production—dawsonite associated with oil shale, coal mine wastes, and alunite. New, large deposits of alunite, overlooked in previous explorations, are now being reported.
All of the aluminum-bearing resources have one feature in common. They lack economic processing methods for alumina production. In the past, the Bureau of Mines published a series of reports on economic and technical evaluations of the known processes for recovering alumina from clay and anorthosite. None of the processes covered was economically competitive with alumina produced from bauxite by the Bayer process. While most of the processes were definitely too expensive for further consideration, a few warranted closer attention because their costs were not appreciably higher than that of the bauxite-Bayer route especially if the cost of imported bauxite increases.
Results of concurrent research, in the Bureau and elsewhere, on variations of the most promising acid processes suggest that economically viable methods to recover alumina from domestic resources may be available, but only on a laboratory scale. Some other processes have had only limited success in pilot plant trials or on a demonstration basis. Before an all-out effort can be made on a large scale to develop prototype or demonstration plants for treating domestic resources, information will be required for rational decision making. The better processes should all be tested on a common basis to supply cost and engineering data.
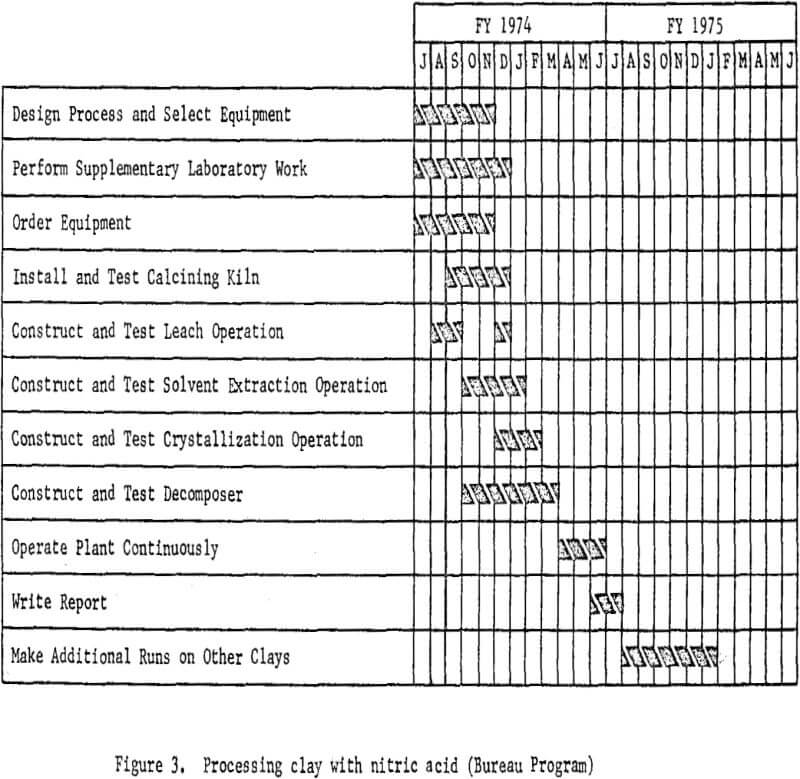
Test Program
On July 1, 1973, the Bureau of Mines began a project at its Boulder City (Nevada) Metallurgy Research Laboratory with a small pilot plant or miniplant to test on a common basis the several processing techniques that yield alumina from domestic resources. The objective of this project, funded at $0.4 million for fiscal year 1974, is to provide the engineering and cost information that will serve as the basis for selecting the alumina processing technology that should be developed in a large-scale pilot plant.
Four domestic aluminous raw materials will be included in the miniplant test program: clay, anorthosite, alunite, and dawsonite. Interestingly, the latter two were not considered significant alumina resources as recently as 1967. Each of the four raw materials is now known to constitute extensive reserves of aluminum, but each presents processing problems. The aluminum content of clay is readily dissolved after dehydration of the hydrous aluminum silicate minerals. It is easily separated from the resulting gangue, but separation from the acid-soluble iron that also occurs in most clays is a problem that has yet to be solved economically. Anorthosite, on the other hand, presents the problem of efficient separation of alumina from silica. Because of the high content of acid-consuming constituents, such as calcium and sodium, in anorthosite, alkaline processing is required, and silica not iron becomes a soluble component. Overlooked reserves of alunite now make it necessary to determine the best processing technique for this mineral that is a sulfate of both aluminum and potassium. Discovery of dawsonite, a basic carbonate of aluminum and sodium, in parts of the major oil shale deposits has focused attention on methods to recover alumina simply from retorted oil shale.
The miniplant operation is designed to produce 25 pounds of alumina per hour. A modular design will allow easy switching from one processing system to another. For ease of operation, the testing program was sited at the Bureau’s Boulder City facility where adequate floor space and laboratories are available. As much equipment as possible has been transferred from other Bureau laboratories to avoid both high purchase prices and current long delivery times. Trained Bureau personnel are available at Boulder City to install and operate the miniplant modules.
Nitric acid processing of clay is being investigated first. This decision was based on attractive preliminary cost estimates, the recommendation of a National Materials Advisory Board Panel (Processes for Extracting Alumina From Nonbauxitic Ores, Report NMAB-278, National Academy of Sciences—National Academy of Engineering, Washington, D.C., Dec. 1970, 88 pp.), and discussions with industry representatives. Hydrochloric acid processing of clay will be the subject of the second phase of the test program for much the same reasons. The raw materials for these tests are already in hand. About 40 tons of clay, typical of the extensive kaolinitic clay deposits in Georgia, is being tested first; eventually, samples of clay and clay-shale raw materials typical of other geographical areas will be tested.
Because of extensive U.S. anorthosite deposits, the lime-soda sinter process has been selected for testing in the third phase of the alumina project. This process appears to have most promise, but additional work needs to be done to solve the serious problem of gel formation after leaching.
Recovery of alumina from clay by sulfurous acid leaching appears to have considerable merit but is in need of further development. A study of this process will be undertaken as a fourth phase of the program, following more detailed laboratory investigations in the early part of the test program.
A fifth phase of the program will focus on alunite. Extensive reserves of this mineral have been discovered since Information Circular 8335 was published in 1967. Even though aluminum salts are being recovered commercially in a small plant in Mexico, the best method to produce metallurgical-grade alumina has yet to be determined.
The final phase of the test program will be concerned with processing techniques for retorted oil shale containing dawsonite. If the retorting temperature is low enough to avoid solid-state reactions that form insoluble compounds, the alumina in the dawsonite can be leached easily with dilute caustic solution. This phase of the program is last in order to allow time to develop the oil shale deposits, to determine optimum retorting procedures, and to obtain a meaningfully large sample of raw material.
At the conclusion of the six-part miniplant program, enough technical, economic, and engineering data should be available to form an engineering judgment regarding the most viable process for further testing in a large pilot plant capable of producing 50 tons per day of high-grade alumina.
Table 1 summarizes the present and projected sources of alumina,
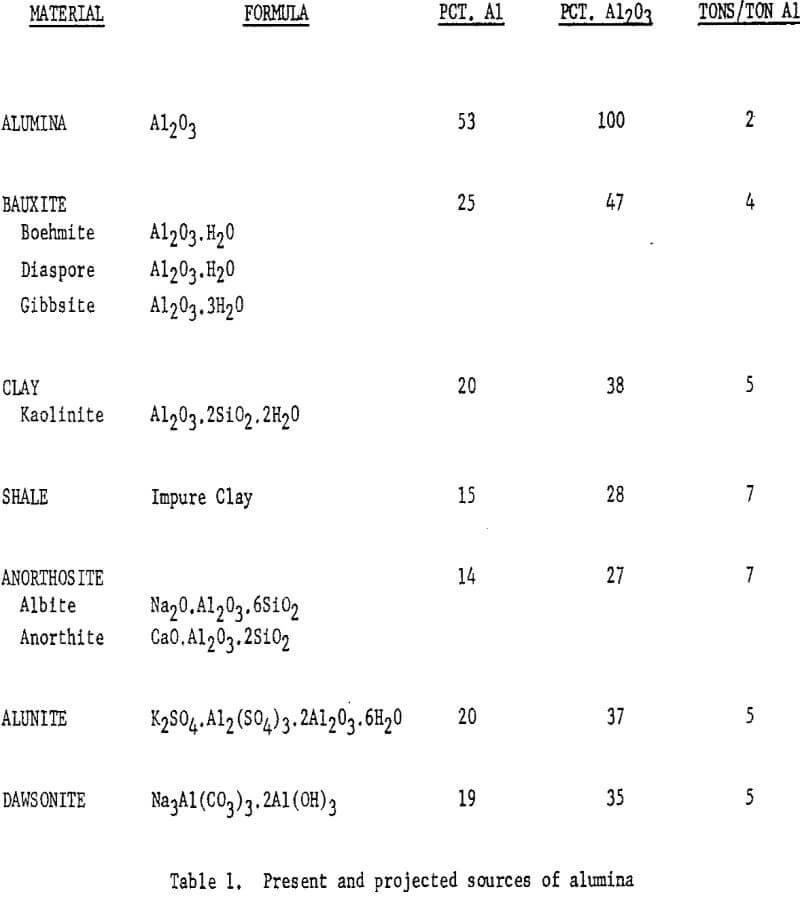
with the compositions of the mineral substances and the requirements per ton of aluminum produced.
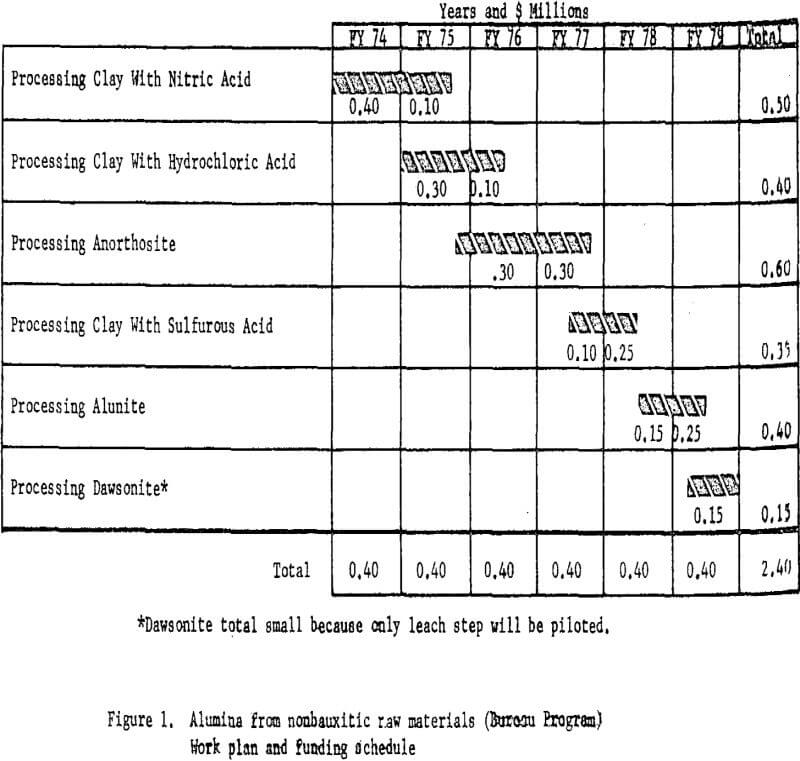
Bureau of Mines Funding and Timing
Based on the starting scale and scope of operations, the proposed alumina test program would have required $0.4 million a year for 6 years for a total of $2.4 million. Funding and timing for each phase of the proposed program are shown in figure 1. The funds and timing include only work done at Boulder City. Other separately funded work, including process engineering and cost estimating, will be carried out at the College Park (Maryland) and Salt Lake City (Utah) Metallurgy Research Centers. The cost of these supporting functions is estimated at about $100,000.
Bureau of Mines Program Phases
Prior investigations on the technology of each of the proposed program phases have been reported in the extensive alumina process evaluations that have originated at our College Park Metallurgy Research Center. Of immediate interest now is IC 8648, Revised and Updated Cost Estimates for Producing Alumina From Domestic Raw Materials, by F.A. Peters and P.W. Johnson.
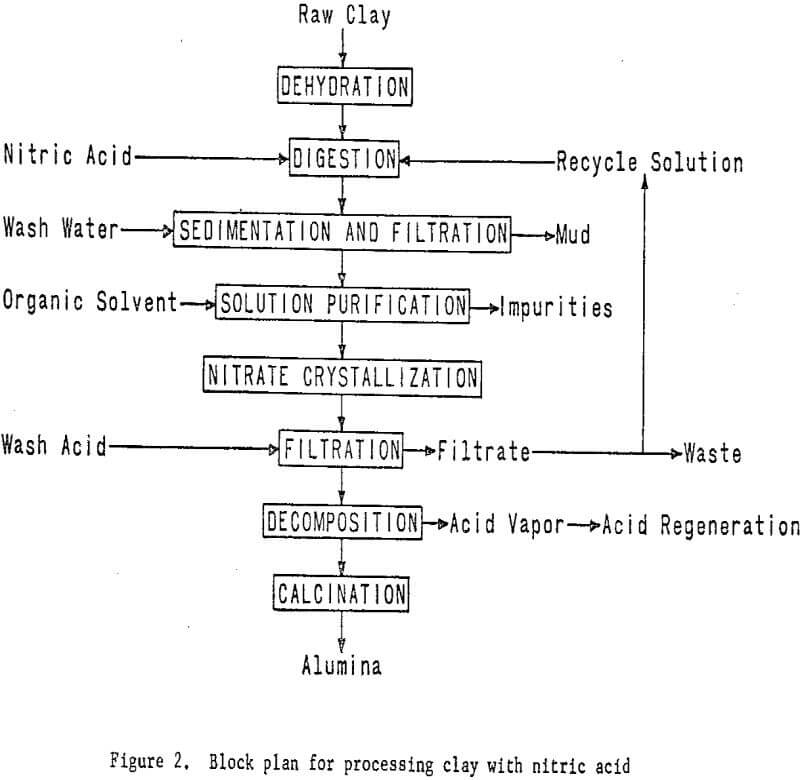
Processing Clay With Nitric Acid
A miniplant was designed and constructed to process approximately 70 pounds per hour of raw clay by nitric acid leaching. Kaolinitic clay is dehydrated in a rotary kiln at a temperature of about 700° C to convert the aluminum components to the acid-soluble form. This step is common to all the acid extraction processes. The dehydrated clay is leached with
nitric acid to extract the aluminum as aluminum nitrate. Iron nitrate, which is an impurity in the leach solution, is removed by liquid-liquid extraction. Aluminum nitrate nonahydrate is then crystallized from the purified solution, and the mother liquor is recycled. A small portion of the mother liquor is bled off to control impurity buildup.
The aluminum nitrate nonahydrate crystals are thermally decomposed under properly controlled conditions to give a hydrated aluminum oxide, which is calcined to alumina, and to recover nitric acid for recycling. Removal of the iron effectively and at reasonable cost and decomposition of the aluminum nitrate are major problems in the process. The latter problem appears to have been solved by the first year’s work. If decomposition is improperly carried out, oxides of nitrogen will be formed rather than nitric acid, thereby increasing processing complexity and costs.
Equipment design and layout are flexible enough to permit thorough testing and optimization of each step in the process before incorporation into the overall process. Individual unit operations can be changed or modified to optimize the overall process. Other types of clay and various kinds of shales can be evaluated as feed materials with little or no change in the equipment.
As data are collected from operation of the miniplant, economic evaluations will be made to guide development of the process.
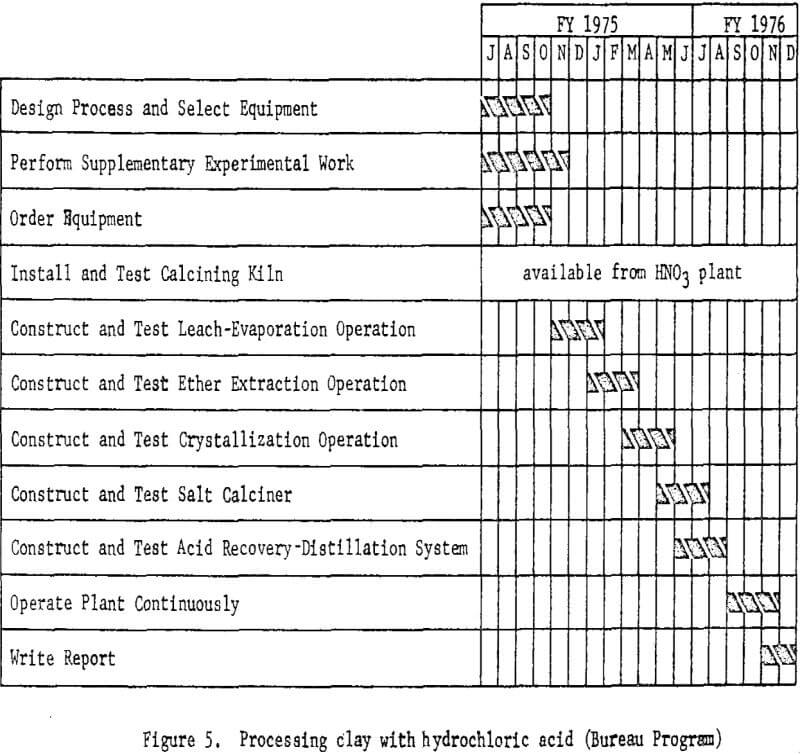
The essential steps of the processing technique are shown in figure 2. Work elements and timing are shown in figure 3.
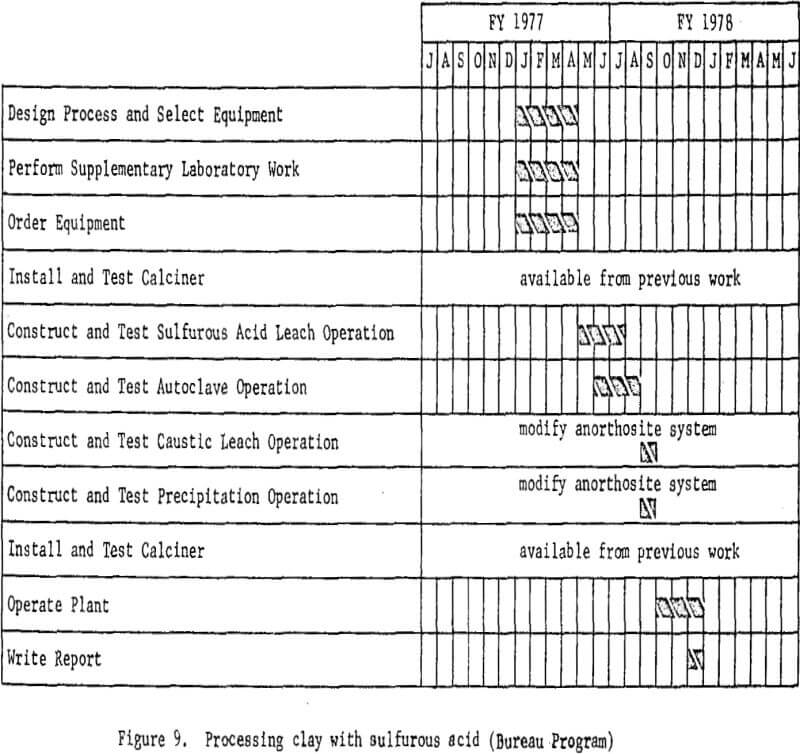
Processing Clay With Hydrochloric Acid
The second phase of the project is to design and construct a mini-
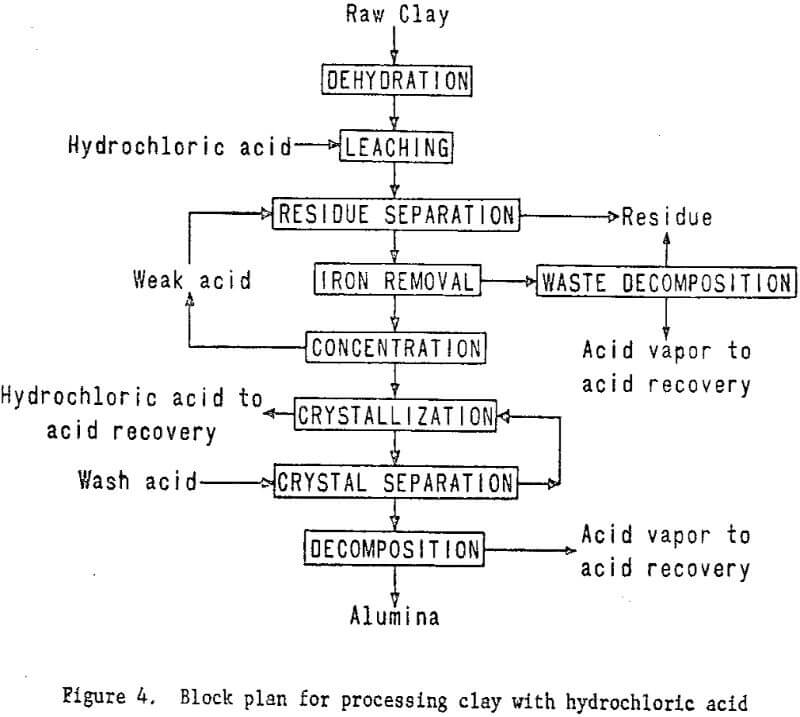
plant for processing clay using hydrochloric acid leaching. Assembly of equipment for the plant is starting and will proceed concurrently with the nitric acid leaching program; subsequently some sections of the nitric acid miniplant, such as clay preparation, will be incorporated into the system. Because hydrochloric acid requires different materials of construction, new equipment will be required for handling solutions. Removal of iron from the leach solutions will be a problem for investigation and presents the greatest opportunity for cost savings. In addition to refining the leaching and slurry handling steps, various iron removal techniques will be investigated.
In this system, dehydrated clay will be leached with hydrochloric acid, and the aluminum dissolved as aluminum chloride. The soluble iron chloride then will be removed from the leach solution by several techniques, including ether extraction and ion exchange. Aluminum chloride hexahydrate will be crystallized from the purified solution and other soluble impurities which will remain in the mother liquor. The hydrated aluminum chloride crystals then will be thermally decomposed in a rotary kiln to produce alumina and recover hydrochloric acid for recycling.
The plant flowsheet will be flexible enough to test technologic innovations and make engineering changes as required. Economic and process evaluations will be made concurrently with the plant tests to provide guidance. The essential steps of the processing technique are shown in figure 4. Figure 5 shown work elements and timing.
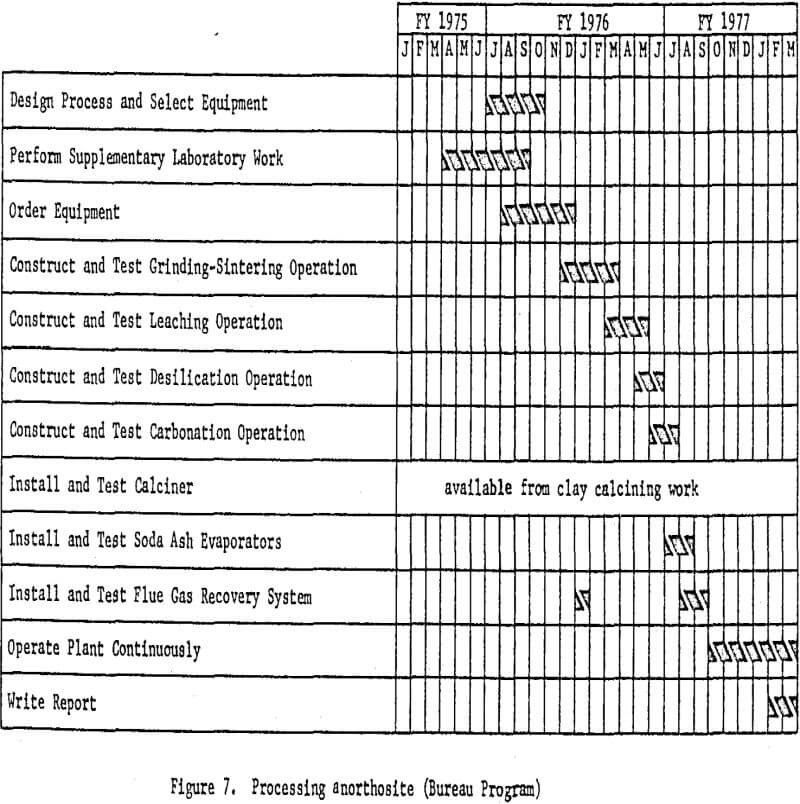
Processing Anorthosite
Anorthosite will be used in the third phase of the program, and the miniplant will be redesigned and altered to accommodate the lime-soda
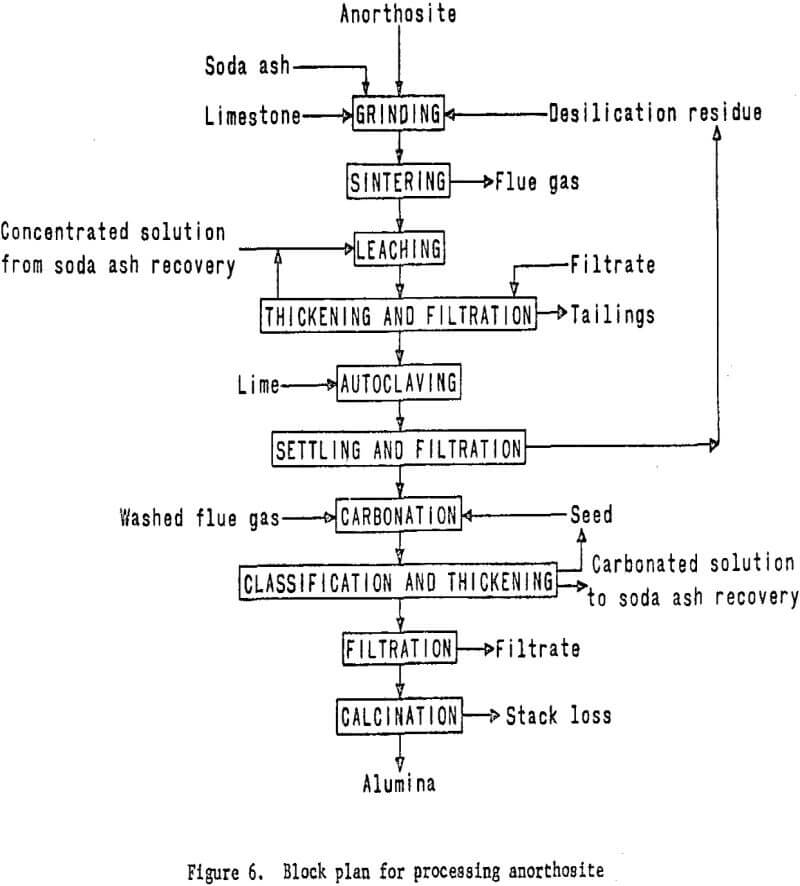
sinter process. The formation of a portland cement-type gel in the leaching circuit, which results in processing difficulties and low aluminum recovery has been a problem encountered by past investigators. Recent information, however, indicates that gel formation can be effectively prevented by carefully controlling raw material ratios and the calcining conditions employed.
The process will involve mixing finely wet-ground anorthosite with limestone and soda ash, followed by sintering. The sinter will then be leached with sodium carbonate solution to dissolve the aluminum as sodium aluminate. Treating this solution with lime under controlled temperature and pressure conditions in an autoclave will precipitate sillceous impurities from the solution.
The purified aluminum-bearing solution then will be treated with carbon dioxide to precipitate aluminum hydroxide. Soda ash will be recovered from the filtrate and recycled to the sintering step. The aluminum hydroxide will be calcined to alumina in a rotary kiln. The essential processing steps are shown in figure 6. Work elements and timing are shown in figure 7.
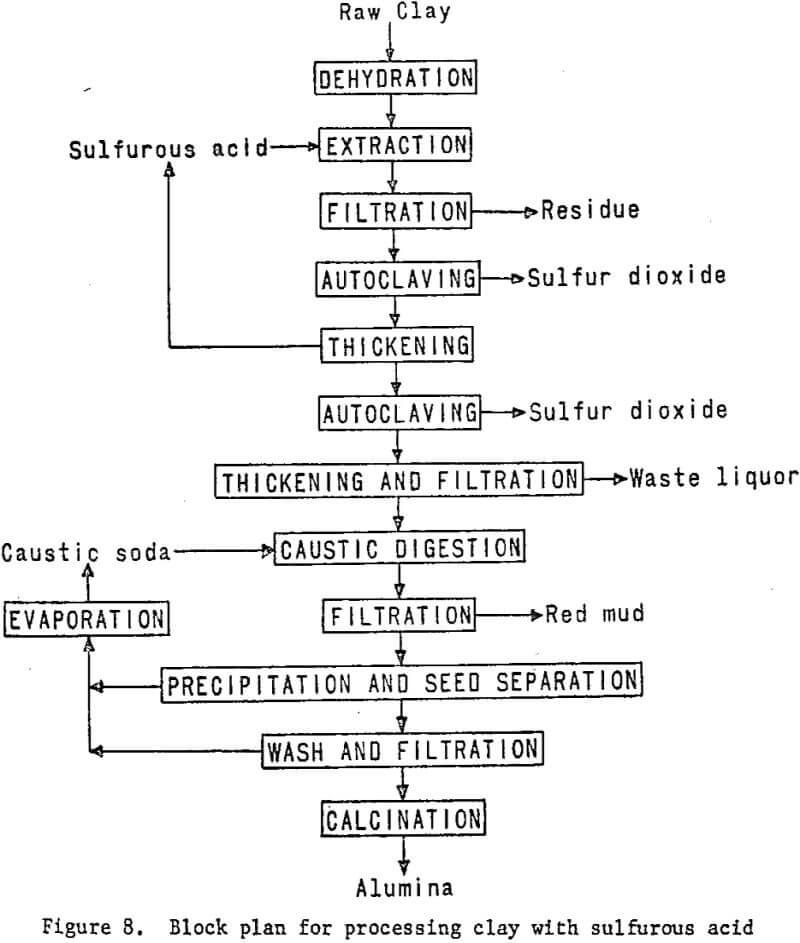
Processing Clay With Sulfurous Acid
Sulfurous acid processing begins with dehydrated clay, just as in nitric or hydrochloric acid processing. The clay will be digested with sulfurous acid solution to dissolve the aluminum, and the solution will be filtered from the silica residue. The aluminum sulfite solution recovered then will be autoclaved under carefully controlled conditions of temperature and pressure to precipitate a basic aluminum sulfite, which then will be decomposed to a crude alumina. Sulfur dioxide evolved from the autoclaves will be recovered and recycled. The impure alumina will
be treated in a modified Bayer process to recover a Hall cell grade product. Laboratory work and literature studies indicate that the problem associated with this process is maintaining oxygen-free leach conditions. The presence of oxygen causes rapid oxidation of the aluminum sulfite to sulfate, which does not decompose in the autoclaving steps, resulting in loss of alumina.
The essential processing steps are shown in figure 8. Figure 9 includes work elements and timing.
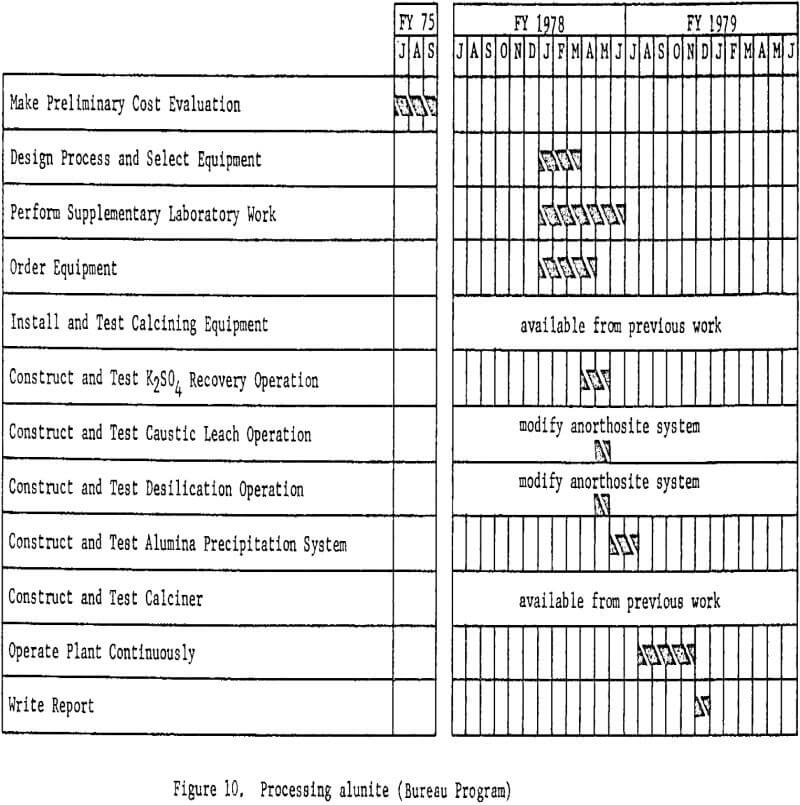
Processing Alunite
Before a miniplant for processing alunite is designed, economic evaluations will be made of various proposed processes. These studies will not be a direct part of the miniplant program, but are necessary for selecting the best techniques for the alunite miniplant. Proposed processes for treating alunite are similar in many respects to those for treating clay; hence much of the required equipment will be available.
An early attempt to recover alumina front alunite was the Kalunite process, developed in the early 1940’s and funded during World War II by the Defense Plant Corporation. A sulfuric acid leach produced potassium alum as an intermediate product, which was thermally decomposed to alumina and potassium sulfate. The latter then was water-leached and crystallized for sale as a byproduct. Sulfur dioxide and trioxide from the decomposition were recovered and converted to sulfuric acid for recycle.
Several yearn ago a Mexican fertilizer company announced plans to build an alunite-processing plant based on a process developed at the University of Guanajuato. In this process alunite is leached with ammonia
to obtain a solution from which ammonium and potassium sulfates are recovered for sale as byproducts. The aluminum-containing residue is successively leached with sulfurous acid and sulfuric acid, and the solutions are combined to precipitate a basic aluminum sulfate. This is then calcined to alumina and oxides of sulfur, which are recycled. Recovery and sale of potassium sulfate and sulfur values are vital to the economic success of any alunite-processing proposal.
National-Southwire Aluminum Company and Earth Sciences, Inc., recently announced plans to build a pilot plant to treat newly discovered alunite deposits near Cedar City, Utah. Details of the proposed flowsheet have not been announced, but it apparently involves leaching to recover potassium sulfate, followed by a modified Bayer process to recover alumina from the leached residue.
A major problem associated with recovery of alumina from alunite could be the residual potassium content of the alumina. Potassium in the electrolyte is believed to cause early failure of the carbon linings of aluminum reduction cells.
Figure 10 shows proposed work elements and timing.
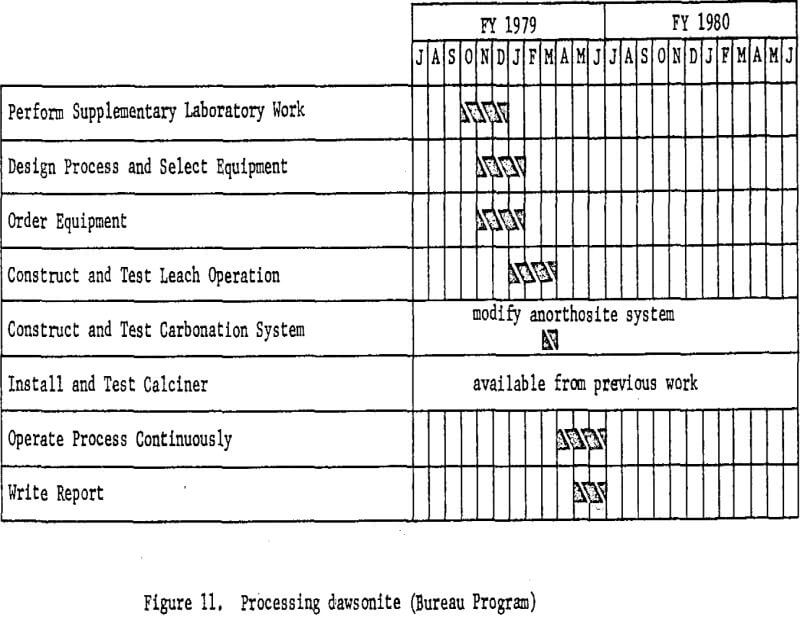
Processing Dawsonite
Dawsonite, one of the raw materials recommended for consideration by the National Materials Advisory Board Panel (Report NMAB-278, December 1970), is found associated with the vast western deposits of oil shale. It is a basic carbonate of aluminum and sodium from which sodium carbonate, now in short supply, may be recovered as a byproduct. Availability of large amounts of this dawsonite, however, depends on the development of a U.S. shale oil industry, since there is no way of economically liberating it from the host shale, nor is the mineral available currently
because of its occurrence at depth.
Because of its basic properties, plus the presence of numerous other acid-consuming impurities in the oil shale, only caustic leach methods are applicable to dawsonite processing. As in the case of alunite, several alternative processes have been proposed and partially investigated, but there are not enough data at present on which to base the design of a miniplant. In essence, processing will consist of crushing and grinding retorted oil shale, leaching with dilute caustic solution, filtering, and precipitating aluminum hydroxide with carbon dioxide. The aluminum hydroxide will then be calcined to alumina. Sodium carbonate solutions from the precipitation step will be recausticized with lime, producing calcium carbonate which may be calcined to fresh lime and carbon dioxide for recycle.
Figure 11 shows the basic work elements and timing.
Cooperative Funding and Timing
The proposed program will require 6 years with Bureau funding alone. In view of the urgency of the work as reported by Secretary of the Interior Rogers C. B. Morton on December 18, 1973, industry was asked to join with the Bureau in support of the program.
If additional money were available, the proposed program could be accelerated, and completed by FY 1977 rather than FY 1979. In addition, thorough investigations could be made of processing clay with sulfurous acid and of dawsonite processing. Further, additional raw materials, such as other varieties of clay and coal shales, could be tried in the completed miniplants.
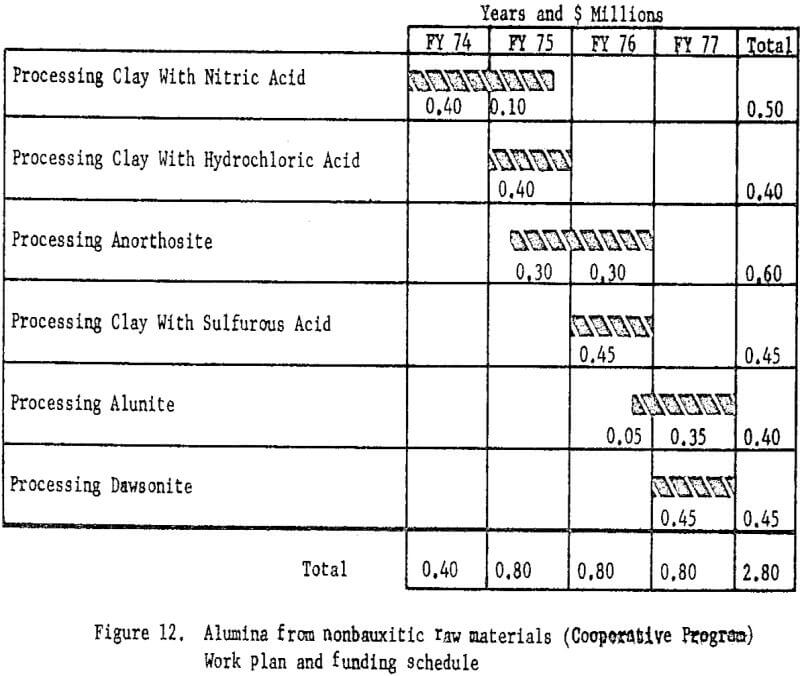
Therefore, the Bureau of Mines proposed that beginning in July 1974 the program be accelerated by increasing the funding to $0.8 million per year for a 3-year period ending June 30, 1977. Industry was asked to provide one-half the funds on a cost-sharing basis. The annual contribution by each participant would be $50,000 annually for the final 3 years of the accelerated program. The total cost of the revised program will be $2.8 million over 4 years instead of $2.4 million over 6 years.
By the end of August 1974 the following firms were participating in the program: Alcan, Alcoa, Araax, Anaconda, Consolidated Aluminum, Kaiser, Martin Marietta, and Reynolds.
Not only will the Nation as a whole benefit from this investigation, but the participants will profit from up-to-date information as the program progresses. Although the Bureau of Mines manages the project, company participation in the program is by the vehicle of a joint steering committee. This committee has the responsibility of reviewing progress of the program, recommending changes, and offering technical guidance through each phase of the work.
The aim of the program is to provide the guidance and technical and engineering data on which to base a considered judgment regarding the process or processes deserving of further development on a demonstration scale.
Funding and timing for each phase of the accelerated program is shown in figure 12.
The essential feature of the cooperative program is acceleration of the phases of the Bureau of Mines alumina test program as well as study in more depth of the processes.
First Phase Activities
The Bureau’s Boulder City Metallurgy Research Laboratory was selected for the site of the alumina miniplant program because ample space as well as experienced personnel were available for the construction, installation, and operation of the equipment. Several important items of equipment were obtained from other Bureau of Mines research facilities in order to expedite the work by circumventing long delivery times quoted for new equipment. The sequence of operations for the nitric acid processing of clay is clay calcining, leaching, iron removal by solvent extraction, evaporation and salt crystallization, and salt decomposition. These operations are described in the following section.
Clay-Nitric Acid Miniplant Construction
The pilot plant was designed to handle about 70 pounds per hour of raw clay, corresponding to about 25 pounds per hour of alumina. The clay initially being tested is typical of the large kaolinitic deposits in Georgia, and analyzed
Al2O3 37.7 percent
SiO2 43.2 percent
Fe2O3 1.18 percent
TiO2 2.77 percent
Loss on ignition 13.5 percent
The clay is calcined in a 14-inch-I.D. by 21-foot-long gas-fired, refractory-lined kiln, with adjustable rotation speed and slope. The temperature is controlled at about 720°C. Calcining drives off water of crystallization and breaks down the mineral structure so the alumina content becomes acld-leachable. The calcine is crushed to minus-20 mesh and screened to remove fines.
Calcined clay is transferred to the leach section in storage hoppers and fed by a screw feeder into an agitated cold-mix tank where it is mixed with 50 percent nitric acid solution. Metering pumps supply concentrated nitric acid, water, and recycle acid at appropriate rates.
The clay-acid slurry flows to a 50 gallon jacketed kettle fitted with agitator and reflux condenser. Hot Dowtherm is circulated in the jacket to start the reaction. The hot slurry overflows to a second insulated, but unheated, reactor and then to a third reactor to allow time for the reaction to go to completion. Slurry then flows to a 36-inch-diameter by 36-inch-deep thickener. Clarified liquor is treated with Separan flocculant and sent to a raw pregnant solution storage tank. Mud from the thickener is sent to an Eimco traveling-belt vacuum filter where the sandy residue is filtered, washed, and discarded. Filtrate is pumped to the raw pregnant solution storage tank, and washings are returned to the leach tanks. These mixed solutions are filtered in a small plate and frame filter press, and the polished solutions are sent to a second storage tank.
The iron content of the original clay, which dissolves along with the alumina, is removed by contacting the clarified pregnant solution with a kerosine solution 0.1 molar in tributylphosphate and 1.0 molar in di(2-ethylhexyl) phosphoric acid. The aqueous solution is first warmed to about 60°C and then contacted with the organic phase in a 5-stage continuous countercurrent mixer-settler system. Aqueous phase flows at 16 gallons per hour and organic phase at 6 gallons per hour. Iron is then stripped from the organic solution in four mixer-settler stages by contacting it with 17 percent hydrochloric acid solution at 5 gallons per hour. The organic phase is washed with water at 21 gallons per hour in two additional mixer-settler units before returning to the extraction section.
Purified pregnant solution is pumped to storage and thence to a 40-gallon jacketed kettle evaporator heated with Dowtherm. Entering solution contains 148 grams per liter Al2O3 equivalent, and the Fe2O3 to Al2O3 ratio is 0.0002. Water is evaporated at a rate of about 4.5 gallons per hour, giving a concentrated solution containing about 175 grams per liter Al2O3. The concentrated solution is cooled to 30°c, whereupon aluminum nitrate nonahydrate crystallizes at a rate of about 40 pounds per hour. A rake classifier is used to separate crystals from the mother liquor. About 10 percent of the mother liquor is discarded to prevent impurity buildup, and the balance is returned to the evaporator. Crystals are washed with 50 percent nitric acid solution in a second rake classifier, drained, and sent to storage. Wash acid is pumped to the leach section. Aluminum nitrate nonahydrate crystals are melted at 70-85°C and pumped to the fused salt decomposer. The latter, the subject of a patent application, is a tank filled with molten potassium nitrate at 340-400°C and fitted with an efficient agitator. Melted aluminum nitrate nonahydrate is fed into the molten KNO3, where it decomposes to nitric acid vapors and alumina hydrate. Nitric acid vapors are condensed and returned to the leach section, while the alumina-containing molten KNO3 is sent to a centrifugal filter. Separated molten KNO3 is returned to the decomposition vessel, while the alumina hydrate is scraped from the filter bowl, washed free of adhering salt, and calcined.
Exhaust ducts over each piece of equipment remove fumes, which are sent to scrubbers, neutralized, and discarded.
Clay-Nitric Acid Miniplant Operation
Following installation of the equipment in each section, test runs were made to insure proper operation of the equipment and to familiarize operating personnel with the processing variables. Shakedown runs were then made on the entire plant, followed by a week-long continuous run to which aluminum company representatives and other interested observers were invited. A summary of the results from that run is presented in the following sections.
Calcining
Feed to the rotary kiln consisted of 26,782 pounds of clay, containing 38.1 percent Al2O3 on a dry basis. The products from the operation were

Available Al2O3 in the usable calcine was 36.1 percent. Dust and fines were saved and will be pelletized for future use.
During an 8-day campaign, only minor troubles were encountered, such as a broken trunnion wheel. Actual calcining time was 176 hours, or 92 percent of available time. Average throughput rate was 152 pounds per hour.
Leaching
The leaching section operated 130 hours out of a possible 152, for an 86 percent availability. Nineteen hours of downtime were to repair the plate and frame filter press, which was allowing fine solids to get into the solvent extraction section and causing emulsification problems.
Solvent Extraction
This section operated 100 out of a possible 103 hours, the downtime being for clean-up of the first four mixer-settler units when solids broke through the polishing filter. The iron content of the pregnant solution was reduced from 1.24 grams per liter of Fe2O3 to less than 0.003 grams per liter for an iron extraction greater than 99.7 percent. Organic loss averaged 11.8 gallons of kerosine-base solvent per ton of Al2O3 produced, however, in the last 25 hours of operation, the average was only 6.9 gallons per ton of Al2O3. This is considerably higher than the economically allowable value for commercial operation but will undoubtedly improve with additional experience. A large part of the loss is due to meeting OSHA requirements for ventilation over the mixer-settler units.
Evaporation and Crystallization
The evaporation unit operated for a total of 111 hours and the crystallizer for 84 hours. The average evaporation rate was 37.1 pounds of water per hour, corresponding to a crystallization rate of about 40 pounds per hour of aluminum nitrate nonahydrate.
Salt Decomposition
The fused salt decomposer experienced mechanical and electrical troubles due to an overheated agitator motor and a salt spill which shorted out a heating element, and hence operated for only a short time. These problems have since been corrected, and the unit is expected to operate properly.
Improvements are now being incorporated into the pilot plant on the basis of the operating experience to date, especially in the solids-liquid separation step of the leaching section, and to provide for washing the aluminum nitrate crystals with nitric acid.
