The reagents now used in flotation consist of various acids or salts, which may be either electrolytes or nonelectrolytes, dissolved in water and some substance or combination of substances, which function as collecting or frothing agents. At times the only dissolved salts present are those naturally occurring in the water used. The general effect of the electrolyte is to greatly sharpen the separation between the gangue and the concentrate. Examples of this are: The use of sulphuric acid with zinc ores; and of sodium carbonate or calcium oxide (lime) with silver-gold ores. Crude pyroligneous acid is also sometimes used when available. Various oxidizing agents, such as permanganates, bi-chromates, etc., are added in the selective flotation of lead-zinc ores. Many other reagents for performing certain specific functions, either real or imaginary, have been proposed, and a number of them have been tried upon a working scale. The wild orgy of experimentation which is now going on in flotation exceeds even that which followed the introduction of the Washoe process for the treatment of Comstock ores, when, among other things, sagebrush tea and tobacco juice were reagents added to the pans. Out of all this will, of course, eventually come a more or less standard practice for the treatment of each class of ore.
Omitting a discussion of the functions of frothing and collecting agents, the reagents used in flotation for this purpose may be classified under five general heads:
- Essential oils;
- fixed or fatty oils;
- alcohols and their combinations with organic acids;
- coal tar and its refined products;
- petroleum and its refined products.
A flotative agent of some kind is required in flotation as now practiced. A single reagent, as, for example, certain, of the essential oils, may perform the dual function of frothing and collecting agent, or a mixture of substances may be required.
In this country, the essential oils used have been wood products, and have been almost exclusively confined to the steam-distilled pine oils. At the present time, the supply of these is limited, and the cost almost prohibitive, so that their use has been dispensed with as far as possible. The oils and tars resulting from the destructive distillation of the various species of the coniferae find a more general use on account of the greater supply and lower cost, although even these have risen in price and the future supply is somewhat problematical if the demand for them in flotation continues to increase at the same rate as in the past.
The fixed or fatty oils, with the exception of oleic acid and crude pyroligneous acid, are scarcely ever used, since they usually give poor results as compared with other flotative agents.
Coal tar is a common and cheap product, but not all coal tars are suitable for use in flotation. Furthermore, the marketing of coal tar is coming more and more into the hands of large distributors, who contract for the output of the various gas plants. The refined products of coal tar are useful flotative agents, but in the case of certain of these, for example, carbolic acid and cresol, the price at present has become- prohibitive. In general, crude coal tar yields the best results when mixed with other oils. It is a frequent practice to use a small proportion of pine oil, in order to modify the froth. Fortunately, in many cases these cheaper mixtures yield very satisfactory results, and can now be obtained at a reasonable cost.
It, appears that only the crude petroleum oils having an asphaltic base are generally useful in flotation. These are invariably mixed with other oils. The refined products of petroleum are usually not satisfactory, except as constituents of oil mixtures. The one exception to this is perhaps the case of kerosene acid sludge, which is a byproduct of petroleum refining. Kerosene acid sludge from eastern refineries gave unsatisfactory results at Anaconda, but California kerosene acid sludge, resulting from the refining of crude oils having an asphaltic base, is satisfactorily used in conjunction with a small proportion of wood creosote for the treatment of Anaconda copper ores. While California kerosene acid sludge is a satisfactory flotative agent with many ores, the available supply tends to limit its use. Formerly California refiners threw it away, but now little of it is available upon the open market, since practically the whole of the present production is contracted for a long time in advance by pioneer users.
A suitable oil supply, both as regards the character of oil to give the best results and its present and future availability, is a matter of serious consequence to companies operating flotation plants. It was with this in mind that I began the investigation of possible sources of flotative agents, with the particular object of affording relief to mines located in the arid regions of the West, where none of the common flotative agents are locally available, and where transportation costs upon those from the outside are high.
Certain plants and shrubs have the peculiar property of secreting oil in the new growth, during the growing season, and particularly in the leaves. Botanists appear to be uncertain regarding the function of this oil in the metabolism of the plant. Some hold that it is a reserve food supply for the plant, while others believe that it is waste product which the plant fails to throw off. Be this as it may, many plants, shrubs and trees contain oil in the leaves and new growth. A good example of this is the case of the eucalyptus tree, from the leaves of which essential oils, which are used for various purposes, are recovered by steam distillation. The leaves of the variety known as amygdalina, unique as being the tallest tree in the world, have afforded a large proportion of the flotative agent used in the concentration of complex Australian ores. This variety of tree is fortunately very plentiful in close proximity to these ore deposits.
In the great arid and semi-arid mining regions of the West the most common of the few plants and shrubs native to the region are the varieties of sage brush known as mountain sage, pasture sage, wormwood sage, etc.; also, in certain regions, greasewood and other shrubs of a similar nature. It is, therefore, to sage brush that my attention has been directed in the search for flotative agents for concentrating Western ores.
It appears that the various varieties of wild sage were first investigated by certain members of the Department of Agriculture, with regard to the possibility of producing from them by steam distillation essential oils suitable for pharmaceutical use. Steam-distilled oil was prepared by Rabak from the variety artemisia frigida in 1905, and during the summers of 1907 and 1908 larger quantities of oil were prepared from specimens of this plant, collected in South Dakota. In 1912, an essential oil prepared by the steam distillation of Ramona stachyoides (black sage) from southern California was also reported by Rabak. This oil was said to contain 40 per cent, of camphor. In 1914, Charles E. Burke and Charles C. Scalione gave an account of an investigation of an essential oil which had been prepared from the same shrub by the steam distillation of several hundred pounds of leaves and twigs collected from brush growing near Riverside, Cal. The yield of oil in this case was 0.9 per cent, of the weight of the material used. This yield was somewhat higher than that reported by the Department of Agriculture. This is perhaps accounted for by the fact that the brush was collected later in the season. This oil is reported as having the following composition:
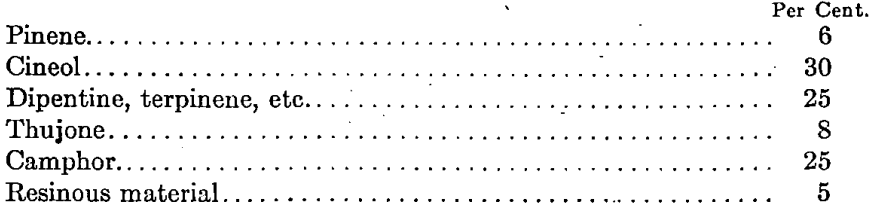
Camphor was separated from a portion of the oil, thus demonstrating the rather interesting possibility of black sage as a source of borneol camphor. Upon request, Mr. Scalione furnished me with a small sample of the original oil. This oil is clear, with a very slight yellowish tinge, and has an agreeable odor. In fact, so far as appearance and general behavior goes, although the chemical composition is somewhat different,
it very much resembles steam-distilled eucalyptus oil from the Australian variety, amygdalina. This oil is a good frothing agent, and it yielded quite satisfactory results upon lead and zinc ores in a qualitative way, although the amount available did not permit of thorough investigation. The idea of investigating sage brush and greasewood as possible sources of flotative agents was conceived early in January, but it was not until early in March that the first 100 lb. of sage brush was forwarded to me, through the courtesy of G. B. Lantz. This lot was collected near Goldfield, Nev., and proved to be the variety Artemisia tridentata. “In the Western Arid Transition zone the flora consists largely of the true sage brush, Artemisia tridentata,” therefore this would be the variety available in the greatest abundance near to the mines of this region (see Fig. 1).
An apparatus for destructive distillation, capable of treating 30 lb. of brush at a charge, was constructed, and two charges of the brush were distilled (see Fig. 2). The products which first came over consisted of acid liquor resembling the crude pyroligneous acid obtained from wood distillation, a black oil or tar, and inflammable gas. Finally these products ceased to come over, but, upon raising the temperature, a consider-

able amount of gas was given off and a rather thick reddish-brown liquor, having an alkaline reaction, began to come over, and with it was a small amount of tar similar to that which came over with the acid liquor. The brown liquor had the characteristic fishy odor peculiar to the amines. There was also at times a distinct ammoniacal odor. The acid and alkaline liquors were kept separate, while the tar from both was combined. These three products were first tried qualitatively in the flotation of finely ground samples of various minerals such as galena, cinnabar, pyrite, etc.
The acid liquor behaved very much as does ordinary pyroligneous acid. The alkaline liquor was a good frothing agent, but the froth carried up little mineral. The tar which came over with the acid liquor proved to be a splendid flotative agent.
Following this, quantitative tests were made upon a number of different ores, employing the tar produced from this lot of sage as a flotative agent. These results, a number of which are given below, were in general very satisfactory.
A sample of zinc ore from the Butte Superior mine, Butte, Mont., containing 22.39 per cent, of zinc, when tested in a Janney laboratory —machine employing a solution of 0.25 per cent, of sulphuric acid, gave 97.0 per cent, extraction of the zinc. The first concentrate contained 53.8 per cent, of zinc, the second 48.9 per cent., the third 40.5 per cent,., and the fourth 18.1 per cent. The oil consumption was at the rate of 0.4 lb. per ton of ore.
A sample of mercury ore from the New Almaden mine, California, containing 0.26 per cent, of mercury, when tested in a Janney machine employing a 0.2 per cent, solution of sodium carbonate, gave an extraction of 90 per cent, of the mercury. The first concentrate contained 3.6 per cent, of mercury, the second 2.5 per cent., the third 1.55 per cent., and the fourth 0.95 per cent. The oil consumption was at the rate of 1 lb. per ton of ore.
A sample of lead ore from the Coeur d’Alene region, containing 12 per cent, of lead, when tested in a Janney machine employing a 0.05 per cent, solution of sodium carbonate, gave an extraction of 92.2 per cent, of the lead. The calculated lead content of the total concentrate was 37.3 per cent. The oil consumption was at the rate of 0.07 lb. per ton of ore.
A sample of silver-gold ore from the Ophir mine, Virginia City, Nev., assaying 0.46 oz. gold and 7.4 oz. silver per ton, when tested in a Janney machine employing a 0.1 per cent, lime or sodium carbonate solution, gave an extraction of approximately 90 per cent, of the silver and 95 per cent, of the gold. The first concentrate assayed gold 5.3 oz. and silver 198 oz. per ton; the second, gold 3.75 oz. and silver 72.9 oz. per ton; and the third, gold 1.32 oz. and silver 35.3 oz. per ton. The oil consumption was at the rate of approximately 0.6 lb. per ton.
The oil consumption when employing sage tar appears to be less than with most of the other oils experimented with in treating the same ores, and the extraction was in general better. Since it is the experience of many that large-scale operation requires less oil than is indicated by small-scale tests, it is reasonable to suppose that the oil consumption in treating the ores cited would be materially lessened when working upon an operating scale, and that possibly the extraction could be bettered. I think the latter is particularly true of the Ophir ore.
Later in March, another lot of brush of the same variety was collected by Mr. Lantz from the same locality, more fully in leaf than that collected earlier in the season. Distillations were run on this lot, keeping the various products separate, so that they could be properly measured and weighed.
Temperature measurements were made at regular intervals by means of a thermocouple, and as a result there was better control of the heating than in former tests. The yield from the last of these tests, which was the most carefully conducted, and was therefore the most representative, was as follows:

The retort was slowly heated for 1 hr. before liquid began to condense. The temperature at the center of the charge at the end of this time, as indicated by the thermocouple, was 60° C.; the temperature at the sides of the charge was probably somewhat higher.
In the next period of 6 hr., during which the acid liquor and most of the tar came over, the temperature rose from 60° to 275° C.; the rise above 100° C. taking place during the last hour and a half.
The alkaline liquor and the last of the tar came over in the last period of 3 hr., during which the temperature rose from 275° to 611° C.
It is reasonable to assume that a yield of about 4 per cent, of the tar oil can be realized if the sage is collected at the proper season and the distillation carried on by the best methods. Then there is the acid liquor, which in certain cases could be used directly in flotation; or it might prove profitable to recover the alcohol, acetic acid, the dissolved tar oil, etc., which it contains. I have not had an opportunity to investigate the alkaline liquor thoroughly, but it would seem to present many interesting possibilities. Among other things, the first lot was found to contain 2.09 per cent., and the second lot 2.18 per cent, of nitrogen. I suspect that this liquor may also contain phenolic bodies which would be useful in flotation when separated from the other constituents. The charcoal is fine, but it should be possible to utilize it for fuel in heating the retorts. One peculiarity is the high percentage of ash which it contains (10.5 per cent, in the one sample analyzed). This may be in part due to dust upon the sage brush, although the brush was chopped fine before distillation, and it would seem that a good deal of the dust would be shaken from it during this operation. If the charcoal were burned, as previously suggested, the alkaline ash might serve instead of lime or sodium carbonate in cases where flotation in alkaline solution was practiced.
The inflammable gas would, of course, be burned under the retorts. The proportion of the heat necessary for carrying on the operation which could be realized in this way is problematical; however, it is reasonable to assume that a considerable part of that required for destructive distillation could be produced by burning the gas and charcoal. Moreover, another interesting possibility in connection with the heating of the retorts is the utilization of the waste heat from various metallurgical operations.
Through the courtesy of Charles Scalione, several ounces of steam- distilled essential oil was prepared from the tip ends of a portion of the last lot of sage brush. Approximately 1 hr. was required for distilling each charge of 20 lb. The yield amounted to 0.43 per cent, of the whole plant. This oil had a greenish-yellow color when first distilled, but became yellow upon standing. It has the characteristic penetrating odor of sage, and a very decided tendency to creep up the sides of the glass containing vessel. This oil appears to have distinctly different properties from the essential oil resulting from the steam distillation of the black sage. If gives promising results with some ores, but in my opinion it is not nearly so good as the tar oil resulting from the destructive distillation of the same shrub. In fact, under ordinary circumstances, I do not think that the steam-distilled oil can be given serious consideration, since the mines that would be most benefited are generally located where fuel is high, and the yield of oil is rather small, probably less than 1 per cent. In addition, there are no other valuable products, unless the tannin extract resulting during steam distillation should have a market value or a demand should arise for “sage tea.”
Time has not been available for making analyses and a study of all the products resulting from the destructive distillation of sage. However, sufficient work has been done to show at least that the light tar is an efficient flotative agent for a considerable number of ores, as indicated by the tests cited.
The idea of utilizing sage brush in metallurgy is by no means new, as is shown by the following quotation, referring to early development of the Washoe process, and the wild riot of experimentation accompanying it:
“ The native sage brush, which everywhere covered the hills, being the bitterest, most unsavory, and nauseating shrub to be found in any part of the world, it was not long before a genius in charge of a mill conceived the idea of making a tea of this and putting it into his pans. Soon, the wonders performed by the sagebrush process, as it was called, were being heralded through the land.”
Considerable interest has recently been developed in sage-brush oil because of its possible utilization as a flotation agent in the mining industry. A list of some of its physical properties, together with the method used in its extraction, may prove of interest at this time.
Something over a year ago, a study of the essential oils in desert plants was begun in the Chemical Laboratory of the University of Nevada. None of the oils so far studied possess properties of special interest to engineers, except the oil of sage, Artemesia tridenlate, which has exceptional power as a flotation agent. This plant, known as common sage brush, also called black sage, is widely distributed over the semi-arid West, being found quite generally on most of the dry plains and mountains west of Missouri.
The method of extracting the oil followed in these experiments is very simple. The leaves, twigs and small branches are placed in an air-tight drum, having a capacity of about 27 cu. ft. Steam is admitted through a number of small openings at the bottom of the retort, and the pressure maintained at 20 to 25 lb. per sq. in. for 3 hr. The escape of the steam from the retort is regulated by allowing it to pass through a stop-cock into a condenser. The water in the receiver is drawn off from time to time and the oil, which is insoluble and floats upon the water, is thus collected. At the end of 2 hr. most of the oil has been driven out, though traces continue to come over for a much longer time. By raising the pressure, the time required could probably be shortened and the yield increased, but the lack of laboratory equipment has prevented the carrying out of this experiment.
The stock wood, bark and branches contain no oil, the distribution of the oil being limited to the leaves and young shoots. There is a seasonal variation in the amount of oil contained. Samples collected on different dates gave the following amount of oil: May 1, 0.42 per cent.; May 27, 0.6 per cent.; June 30, 0.72 per cent.; Aug. 1, 0.9 per cent.; Sept. 10, 1.0 per cent. The increase appears fairly constant from early spring, when the leaves first appear, until light frosts occur in the autumn. When the plant is air-dried there is some loss of oil, as the following data will show: Two 100-lb. samples were collected at the same time. One was distilled when green; the other was air dried for 10 days before distillation. The green sample yielded 275 grams, and the dried sample 248 grams of oil, showing a loss of about 10 per cent.
A laboratory experiment can furnish little data useful in forming an estimate of the commercial cost of production. A man working for 6 hr., and using a pair of common pruning shears, collected twigs which yielded 1 lb. of oil. Since only a small percentage of the oil is lost if the brush is dried, the most economical method of production would perhaps be to collect it in large quantities, by using a tractor engine and a drag, in some such way as land is cleared for farming. When the brush is dry, the leaves and young shoots are easily shaken from the limbs. Thus the amount of material to be distilled would be greatly diminished and the oil perhaps obtained at a cost and in quantity sufficient to make it available as a flotation oil, if not alone, possibly as an ingredient, to increase the flotative power of other oils.
The crude oil is dark in color. When redistilled with steam it is water-white at first, changing gradually to a straw-yellow color upon standing. It has the following physical properties: Density at 15°C., 0.9206. Refractive index at 20°C., 1.4732. Rotation at 20°C., —4.69. At 98°C., a light oil, with a very sharp and pungent odor, begins to distill, but only after the temperature is above 165°C. does rapid distillation take place. At 180°C., the oil turns dark and decomposition begins. At a pressure of 12 mm., and below 125°C, almost all the oil can be distilled.
The chemical properties of the oil are as yet undetermined. There are small quantities of alpha and beta pinene. The main part of the oil has a camphor-like odor and taste, but has failed to give the ordinary tests for ketones. The fraction boiling at 175° to 180°C. has some of the properties of ordinary cineol, but is acted upon by metallic sodium, which indicates that the chief ingredient is not cineol. The chemical composition, which has little interest in this connection, will be worked out later. The important question for the engineer is: Can the oil be produced in quantity and at a cost that will make it available for ore flotation?
