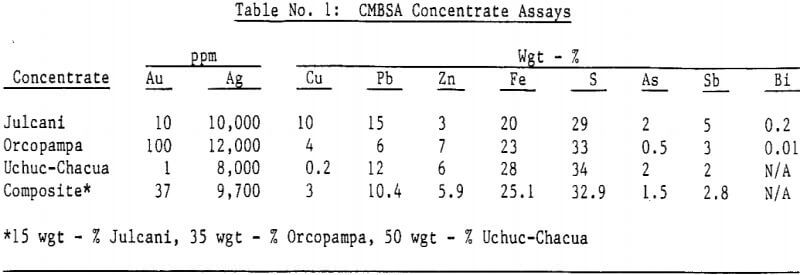
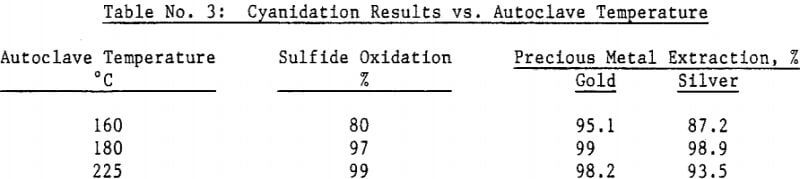
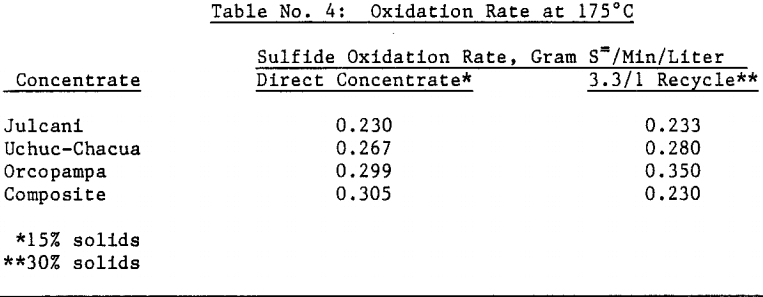
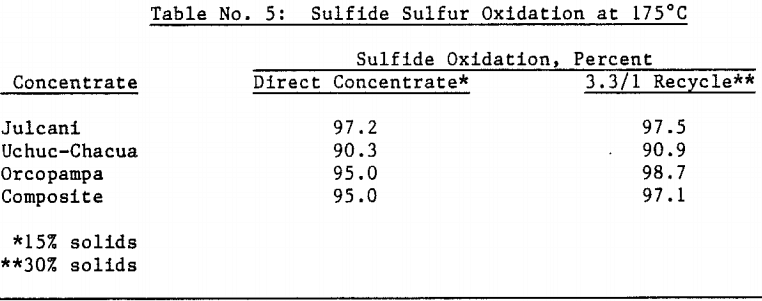
Silver bearing bulk sulfide concentrates from several Peruvian mines were autoclaved in a 19 liter (five gallon) laboratory autoclave. Cyanidation of autoclaved concentrates following a hot lime jarosite destruction step extracted over 98 percent of the silver and over 95 percent of the gold. Autoclave operating parameters such as time, temperature, pulp density and autoclaved solids recycle were investigated and reaction rates determined.
Autoclave oxidation of sulfide flotation concentrates presents a series of problems usually not encountered in whole ore autoclaving. Excessive heat and acid generation results from high sulfide levels in the concentrates. This requires pulp dilution with water or recycled autoclave slurry to maintain an acceptable heat balance.
The following treatment steps were delineated during testing,
– Concentrate Regrinding to 95 wgt – % minus 325 mesh to increase surface area and reactivity of sulfides.
– Preacidification of reground concentrate to destroy carbonates in the autoclave feed, thereby eliminating carbon dioxide gas evolution during autoclaving.
– Autoclaving to oxidize sulfides.
– Washing, Thickening and Filtration of autoclaved slurry to remove solubilized metals and acid.
– Hot Lime Treatment of washed autoclave residue to destroy refractory silver – bearing Jarosites.
– Cyanidation of reacted autoclave residue to extract gold and silver.
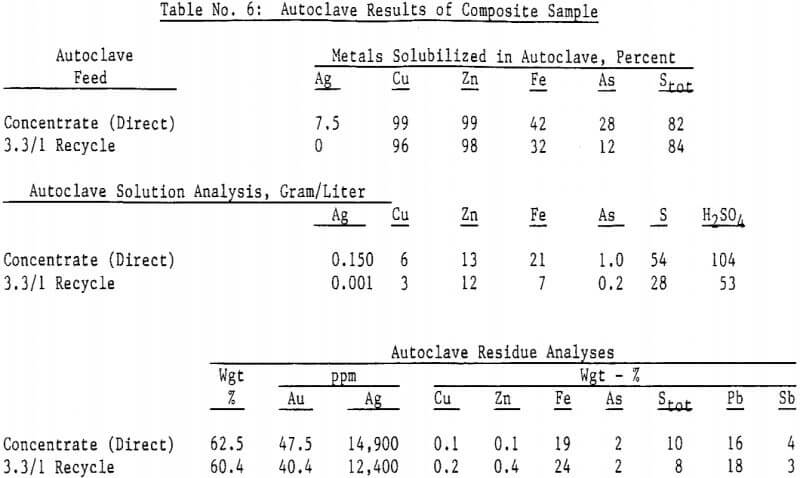
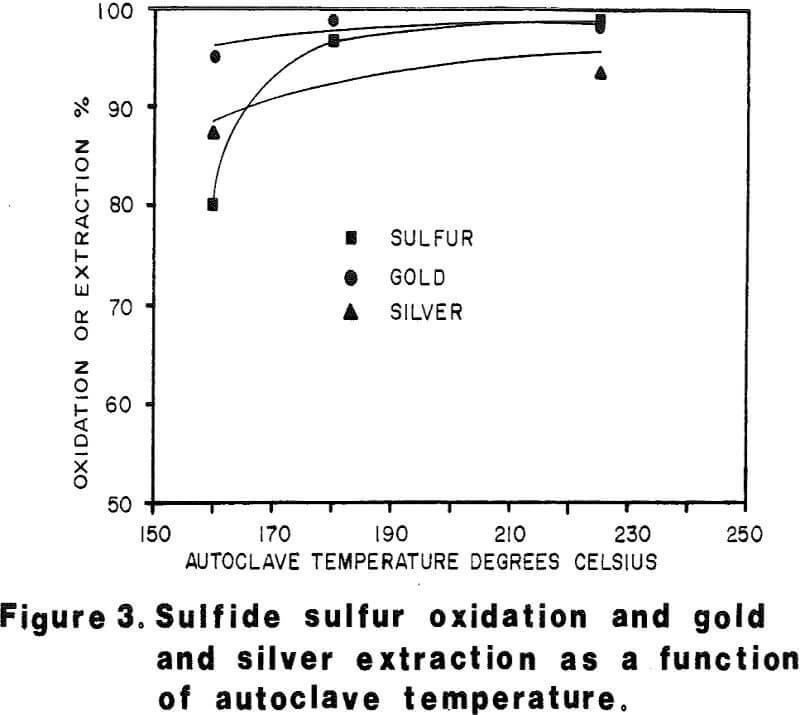
Autoclave temperatures from 160 to 225°C and autoclave feed pulp densities from 5 to 30 percent solids were tested. Initial results indicated that pulp densities above 15 percent (concentrate) solids resulted in incomplete reaction due to limited metal sulfate solubility, particularly copper in the Julcani concentrates. Autoclave tests on undiluted concentrates were subsequently limited to a maximum pulp density of 15 percent concentrate solids. The concentrate – water slurry was acidified to pH 3 in these tests.
An oxidation rate of 0.250 grams sulfur per minute per liter of active volume will require an autoclave of approximately 4 meters (13 feet) diameter and 10 meters (35 feet) length assuming 70 percent active autoclave volume. This calculation was based on 100 tonne (110 ton) per day flotation concentrate feed with a 3.3 part recycle autoclave solids to one (1) part flotation concentrate feed at 30 percent solids pulp density.
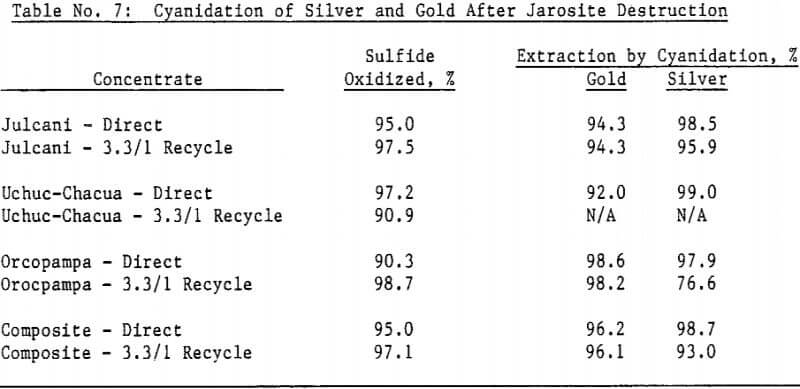
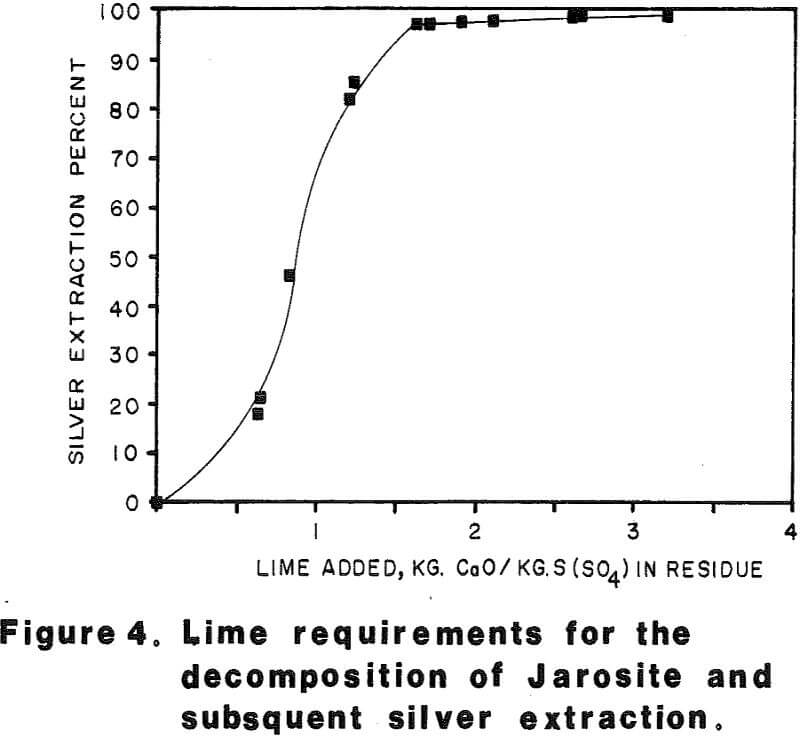
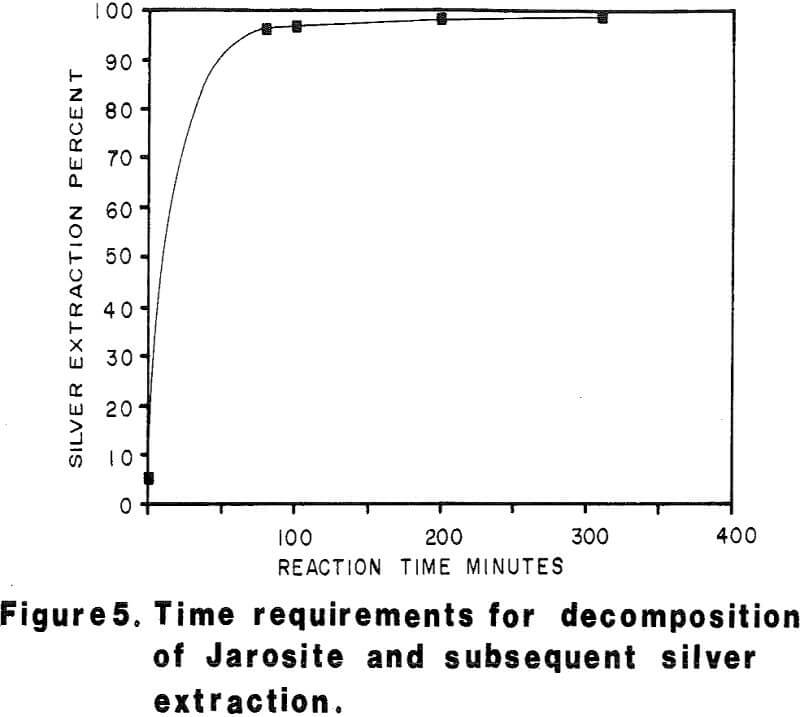
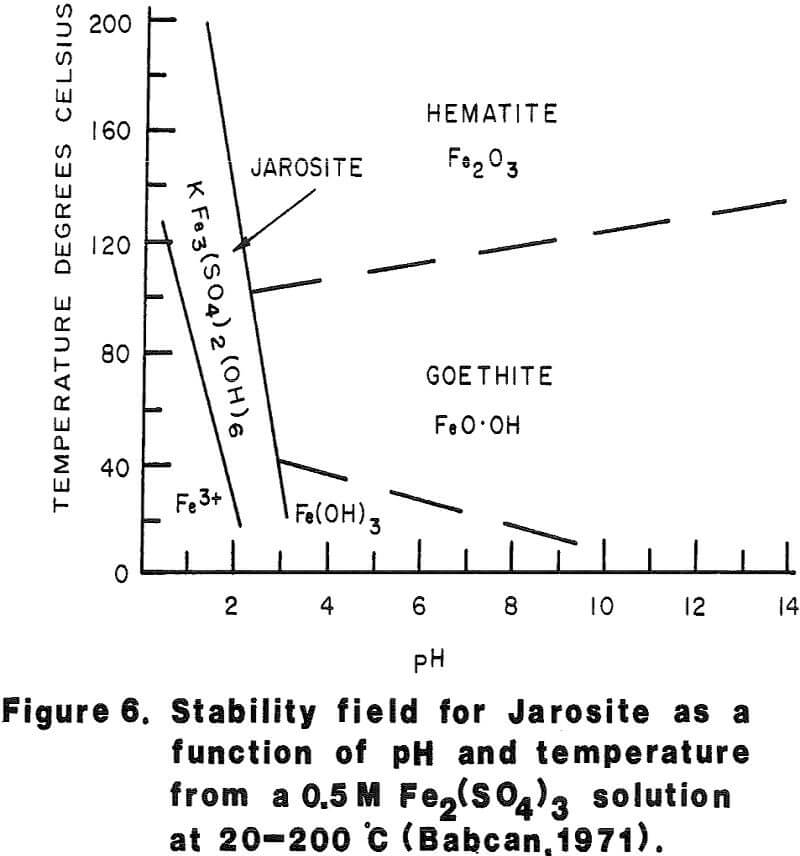
Most of the silver in the autoclaved solids is associated with Jarosites which are formed by hydrolysis of ferric sulfate. The silver associated with these Jarosites is extremely refractory to cyanidation, resulting in typical silver extractions of less than five (5) percent. Jarosites may be decomposed by lime at elevated pulp temperatures of 85 – 90°C.
The amenability of silver to extraction by cyanide after the jarosite destruction step was reduced by recycling autoclaved solids back to the autoclave. Although the reason for reduced silver extraction is not clear at this time, incomplete sulfide oxidation is not responsible, for sulfide oxidation levels were actually higher in the recycle tests. Occlusion of precious metals by elemental sulfur in the autoclave residue may also be excluded due to the high gold extractions obtained by both direct concentrate autoclaving and autoclaved solids recycle.
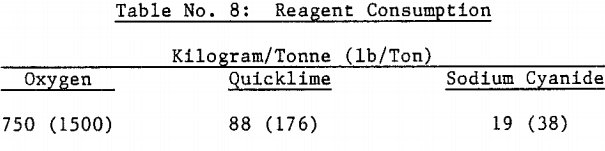
Approximately 530 kilograms of sulfuric acid was produced per metric tonne of concentrate autoclaved. This amount of acid required approximately 500 kilograms of limestone and 100 kilograms of quicklime per metric tonne of concentrate to neutralize the autoclave wash thickener overflow to pH 10 in laboratory testing.
Pressure oxidation followed by jarosite destruction appears to be an effective method for treating selected silver-bearing refractory flotation concentrates prior to cyanidation. However, several problems were encountered during direct autoclave of concentrates, namely:
- Excessive heat generation.
- Moderate silver dissolution in the autoclave.
- Unreacted sulfide pellet formation.