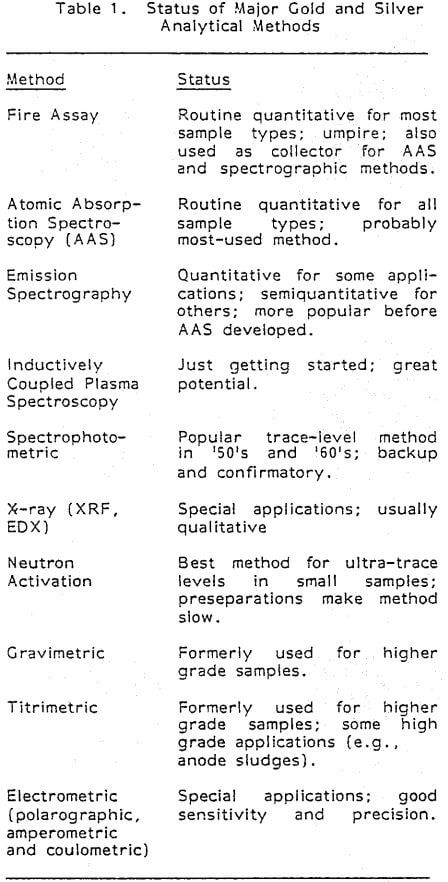
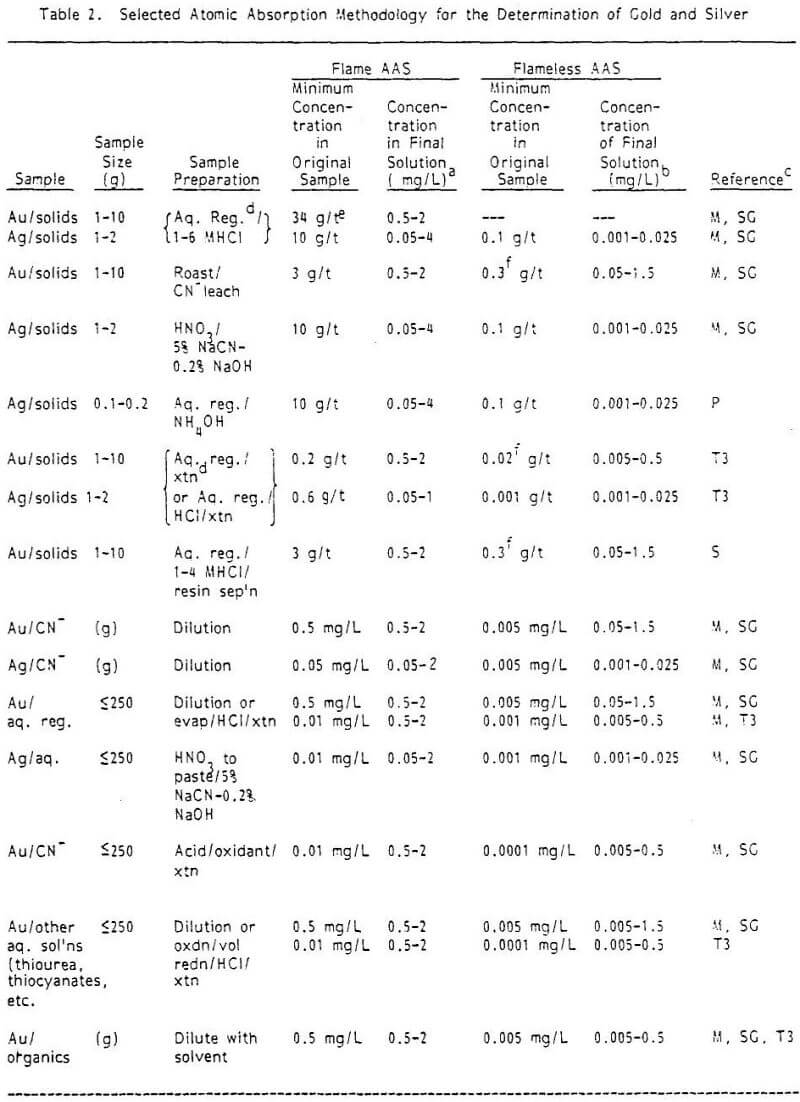
Sample Preparation: Aqua regia with, occasionally, the addition of hydrofluoric acid is the most used lixiviant, and is applicable to most ore types. Other combinations of acids as well as cyanides are also used, and pre-roasting is sometimes applied.
Digestion time and amount of reagent vary, quite naturally, with the amount of acid consuming materials in the sample. Thus, raw ores are usually simpler than base metal and pyrite concentrates. In the latter case, reagents in addition to aqua regia are often added to completely oxidize the sulfide; otherwise, sulfur beads may form on the reaction mixture. However, tests in this laboratory with such concentrates, at low Au and Ag levels, showed that destruction of the sulfur was not necessary in order to achieve recovery of the Au and Ag.
Detailed digestion procedures vary with the sample type, size of gold particulate, and laboratory. No one procedure or even reagent combination is optimum for all types of samples. This was demonstrated within our laboratory, where aqua regia is the standard lixiviant for most solid samples. However, silver in a carbon bed (used to remove the Ag from cyanide leach liquors) could not be dissolved quantitatively with either an aqua regia or a cyanide leach. It was finally dissolved quantitatively after the carbon was completely destroyed with hot, concentrated, HClO4.
After digestion, the sample is redissolved in the solvent from which AAS measurements or solvent extraction is to take place. The sample is usually boiled or baked before the final solution makeup. General consensus is that this is done to remove NOx. The reasons for this are not altogether clear, but it has been reported that NOx interferes with AAS silver readings, and some gold methods also have included baking. However, methods for both Au and Ag are used which do not include such baking, and the excess nitric acid does not interfere with subsequent extraction of gold into MIBK (Ichinose, 1971). Therefore, the general need for baking appears to be an unresolved question at present.
Independent of the above question, it has been reported that boiling samples to dryness and then baking at high temperatures can reduce Au(III) to Au (I) and Au, which do not extract into ketones such as MIBK. Therefore, Tindall recommends that the sample be kept moist and NOx be removed by boiling from, e.g., 50% HCl. Some authors add oxidants such as bromine or permanganate to the final makeup solution to counteract any prior reduction of Au(III).
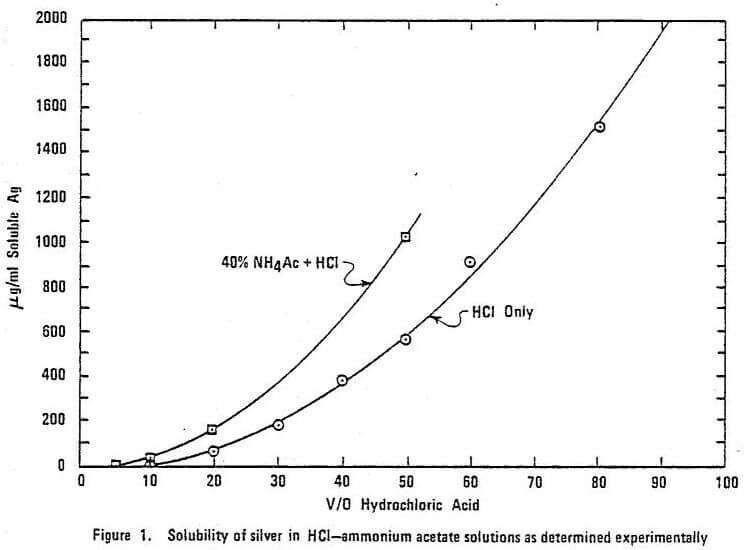
Both AAS measurements and extraction can be performed from HCl or aqua regia solutions, the same components used in digestion. Although Au (III) is sufficiently soluble in even dilute HCl, silver may be lost to precipitation if the HCl is not concentrated enough. Figure 1 gives the solubility of Ag+ ions as a function of HCl concentration. This figure does not take into consideration chloride used in complexing other metal ions such as Fe3+, Al3+, and Pb2+. Common practice is to make the final dissolution in 25 to 50% HCl which, according to the literature, is sufficient to hold at least 50 ppm Ag in solution. Ammonium acetate (NH4Ac) is often added to the HCl solution when silver is determined in the presence of high lead. Although this improves the solubility of silver slightly (cf. Fig. 1) and prevents PbCl precipitation (which can coprecipitate silver), the value of ammonium acetate is not accepted by many.
Leach solutions such as cyanides may be aspirated directly into the flame. Standards should match the samples quite closely both in the case of solution samples and digest-makeup samples. Reliable background correction is essential for quantitative work, especially in the case of gold, unless both the standards and sample matrices are nearly identical. This is difficult to accomplish inasmuch as different samples usually contain different, unknown levels of the base metals and solvents. Background correction is practically a necessity with flameless AAS.
Air-acetylene flames and wavelengths of 328.1 nm (for Ag) and 242.3 nm (for Au) are employed almost universally. Typical sensitivities are approximately 0.05 ppm for Ag and 0.3 ppm for Au.
Solvent Extraction: As Au and Ag concentrations decrease and additional sensitivity is needed, solvent extraction (SX) is usually employed. This serves to concentrate the sample, remove interferences (discussed later) , and enhance the AAS signal for a combined sensitivity increase of up to 200 times. A suitable organic solvent provides quantitative extraction from the digestate or solution sample at a small organic/aqueous ratio, low solubility in the aqueous phase, selective extraction of Au or Ag over the other ions present, a flame transparent at the wavelengths involved, and enhancement of the absorption. The better and more popular solvents in these respects are listed in Table 3. Many others have been studied, however, as evidenced by the more than 100 SX schemes for gold and more than 20 for silver which were published from 1970-80. Most of these references can be found readily in the reviews by Sen Gupta (1973) and Das and Bhattacharyya (1976), and in the biannual bibliographies of Atomic Absorption Newsletter. The great differences in these numbers is no doubt due to the greater sensitivity of the AAS response for silver, coupled with the fact that commercially interesting silver deposits are of higher grade than their gold counterparts.