An acid mine drainage sludge sample containing 3.6% Mn, 0.18% Ni, 0.15% Co and 0.39 % Zn was obtained from the central part of West Virginia and leached with sulfuric acid at pH 3 and at room temperature. Air was bubbled through the leach solution to precipitate the iron content from the solution at pH 4.5. The leach solution contained 258ppm Zn, 105ppm Ni, 90ppm Co and 2050ppm Mn.
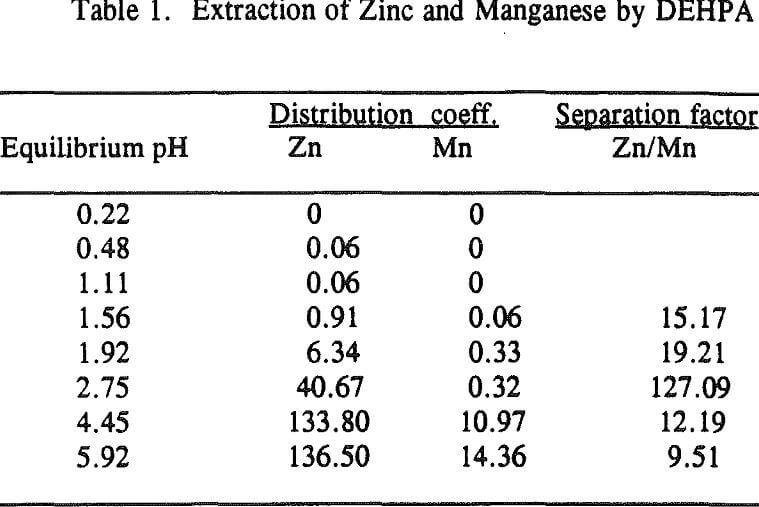
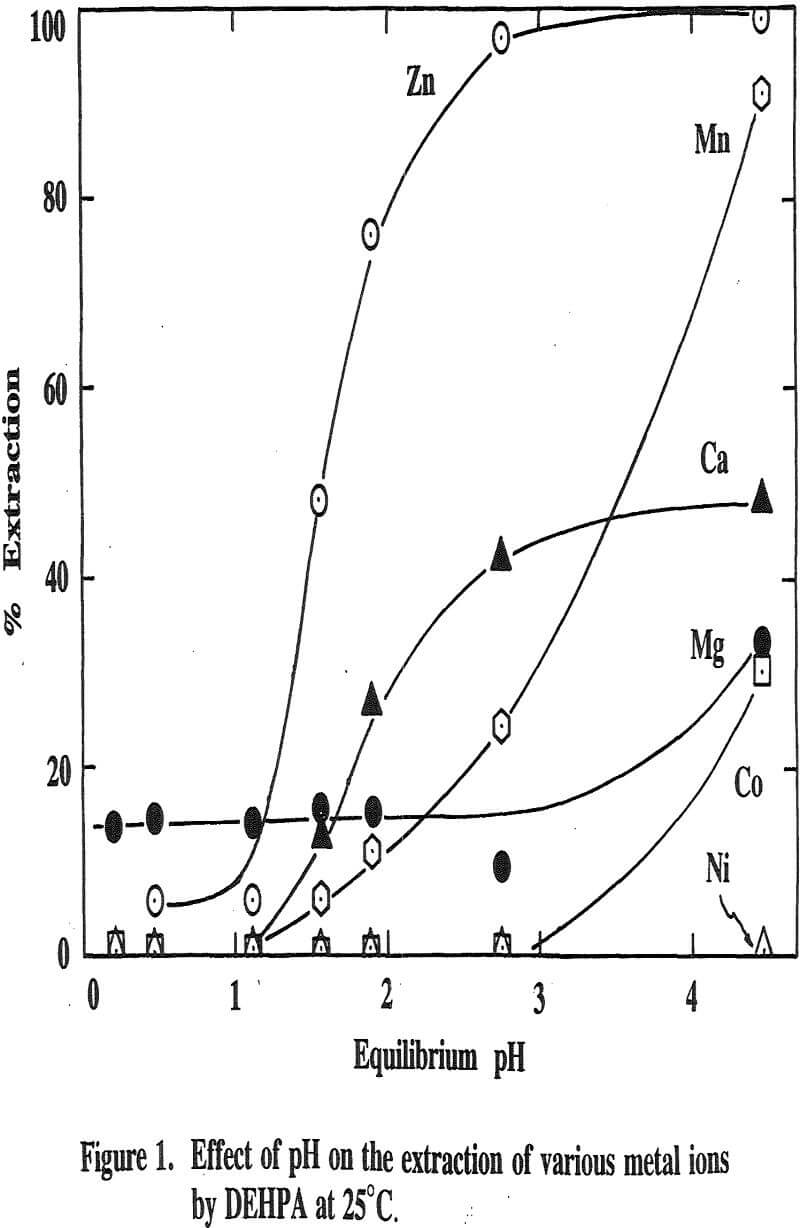
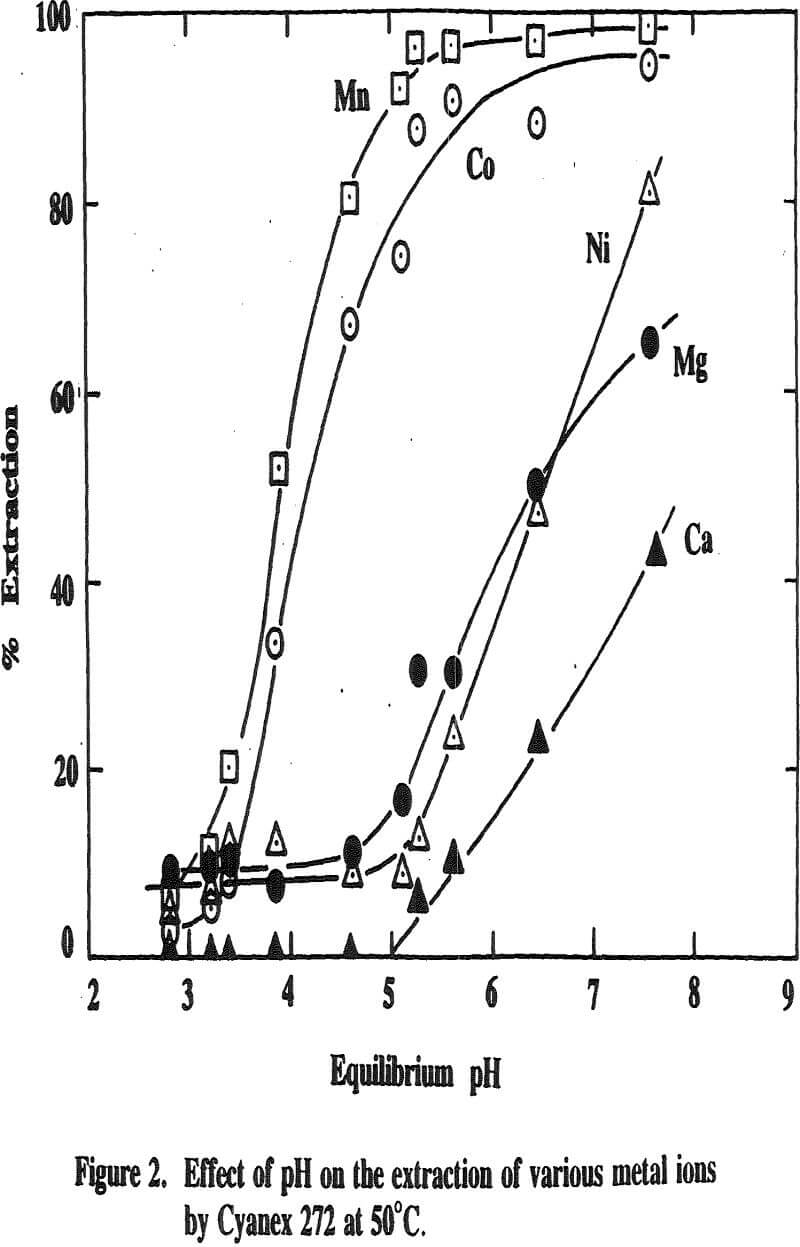
Three distinctive solvent extraction studies were performed to selectively extract these metal ions in a sequential operation with DEHPA, Cyanex 272, and then Lix 84. The A/O ratio was 1 and the extractants were diluted 10 times with kerosene. A study of solvent extraction with DEHPA showed that Zn could be extracted without Ni or Co up to pH 2.75. A pH of 2.75 is considered to be the optimum pH to maximize the selective separation of Zn from Mn.
The selective recovery of various metal ions including nickel, cobalt and manganese from manganese-nodule leach solutions with sulfuric acid was the subject of numerous studies in the past. An excellent review is available in the literature. A study performed by Ando et. al. involved the selective recovery of cobalt from nickel using M2EHPA (PC-88A). They found that the separation factor of M2EHPA was 200 times greater than that of DEHPA (di-ethylhexyl phosphoric acid) which is well known as a cationic extractant.
It was found that the iron concentration was reduced from 5576ppm to less than 0.1ppm through the oxidation step while other metal ion concentrations were only slightly reduced. The concentrations of the metal ions after the iron precipitation step were 258ppm Zn, 105ppm Ni, 90ppm Co, 2050ppm Mn, 4600ppm Mg and 395ppm Ca. This leaching- and-iron precipitation experiment was repeated many times to collect a bulk solution for the subsequent study of solvent extraction.
Using a solution pH (initial pH 2.5 and final pH 1.8) a bulk solution was produced by contacting 800ml of each organic and aqueous phase in four stages and stirring them for 10 minutes. After each stage, the pH of the aqueous was readjusted to 2.5 for the next stage. The analysis of the aqueous phase after the final stage showed that the concentration of Zn was 2ppm, Ni 105ppm, Co 90ppm, Mn 1640ppm, Mg 3800ppm and Ca 160ppm. This result revealed that Zn was extracted by more than 99%, Ni and Co were not extracted, Mn by 20%, Mg by 12% and Ca by 59.5%.
The extraction of magnesium is not a function of solution pH up to 2.75 unlike those of Zn, Ca and Mn which are shown to increase with increasing solution pH. Since the Mg extraction was only 12% with DEHPA in the four-stage operation, as mentioned previously, it appears that the extraction of Mg does not represent the intrinsic extraction but rather is due to some inherent problems (e.g., analysis with an atomic absorption unit) which occurred from this particular system.