Solvent extraction is widely used in hydrometallurgical processing of uranium for purification and upgrading leach solutions prior to recovery of metal values. Conventional solvent extraction processing utilizes mixer-settler units to achieve mixing of the aqueous and organic phases. Use of mixer-settlers permits continuous treatment of feed, but the processing rate is restricted by the time required for phase separation after mixing. Because of the high-shear type mixing used in a mixer settler a portion of the organic phase becomes mechanically entrained in the aqueous resulting in loss of extractant. For this reason application of mixer-settler units is often limited by the settling area required for phase separation, especially for systems where high volume throughputs are desirable or necessary.
Experimental
The aqueous feed solutions were prepared by dissolving purified U2SO4·3H2O in distilled water and adjusting the pH to 1.5 with reagent grade H2SO4.
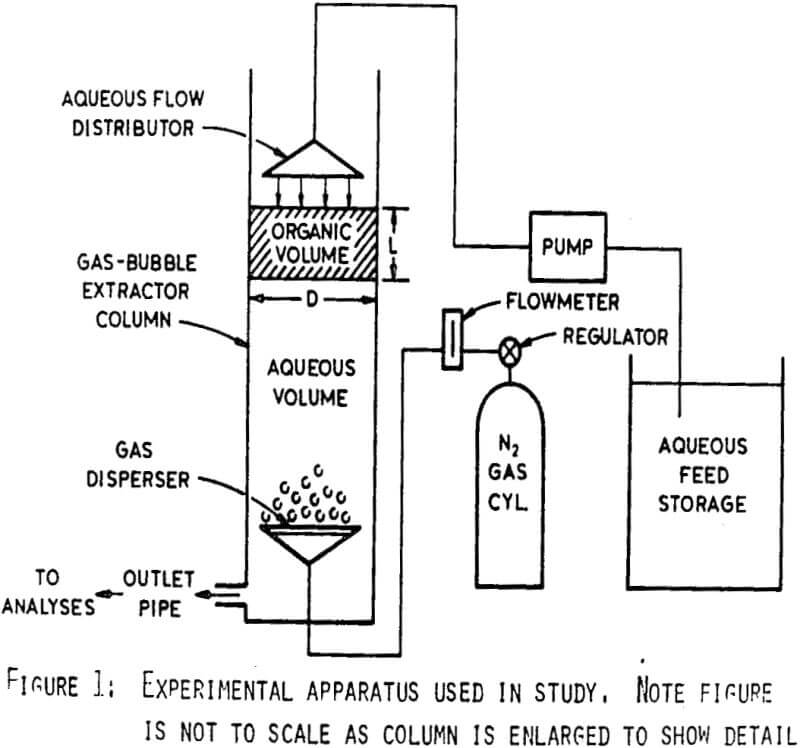
The column was constructed from 5.5 cm I.D. clear plastic tubing. A gas dispersion frit 3.5 cm in diam with 30 µ diam pores was mounted 10 cm from the bottom of the column. Nitrogen gas admitted through this frit provided the mixing. The flow rate of N2 gas was measured with a flow meter and controlled by a regulator. Aqueous solution was pumped at a controlled rate from a storage reservoir using a variable speed peristaltic pump and distributed as a gentle spray at the top of the column.
Periodic samples of barren aqueous, as well as head and total raffinate samples, were analyzed for U3O8. Organic loss was determined by measuring the volume of organic before and after each test. Each test was terminated when the organic phase became saturated with uranium. A mass balance was performed using the total volume of raffinate and the concentrations of U3O8 in the feed and raffinate to determine the organic loading.
Results and Discussion
Three factors permit operation of the column as proposed. First, the density of the organic phase is less than that of the aqueous, so it will naturally rise in the column. Second, the countercurrent flow of aqueous solution and gas bubbles produces mixing of the organic and aqueous phases without the high degree of dispersion obtained in high-shear type mixing. Thus, a thorough dispersion of organic and aqueous is not produced throughout the column and phase separation is more readily achieved. Lastly, since the organic phase is surface active, organic droplets adhere to gas bubbles as they rise and are carried to the top of the column. The above factors increase the probability of the organic phase remaining in the upper portion of column during operation. As a result, aqueous may be continuously processed to recover uranium with minimal organic loss
Consequently, uranium extraction occurs in the region above the gas disperser while the region below permits separation of the organic and aqueous phases before the aqueous leaves the column. Thus, pregnant aqueous may be fed to the top of the column and barren aqueous withdrawn from the bottom providing continuous extraction of uranium with little loss of organic.
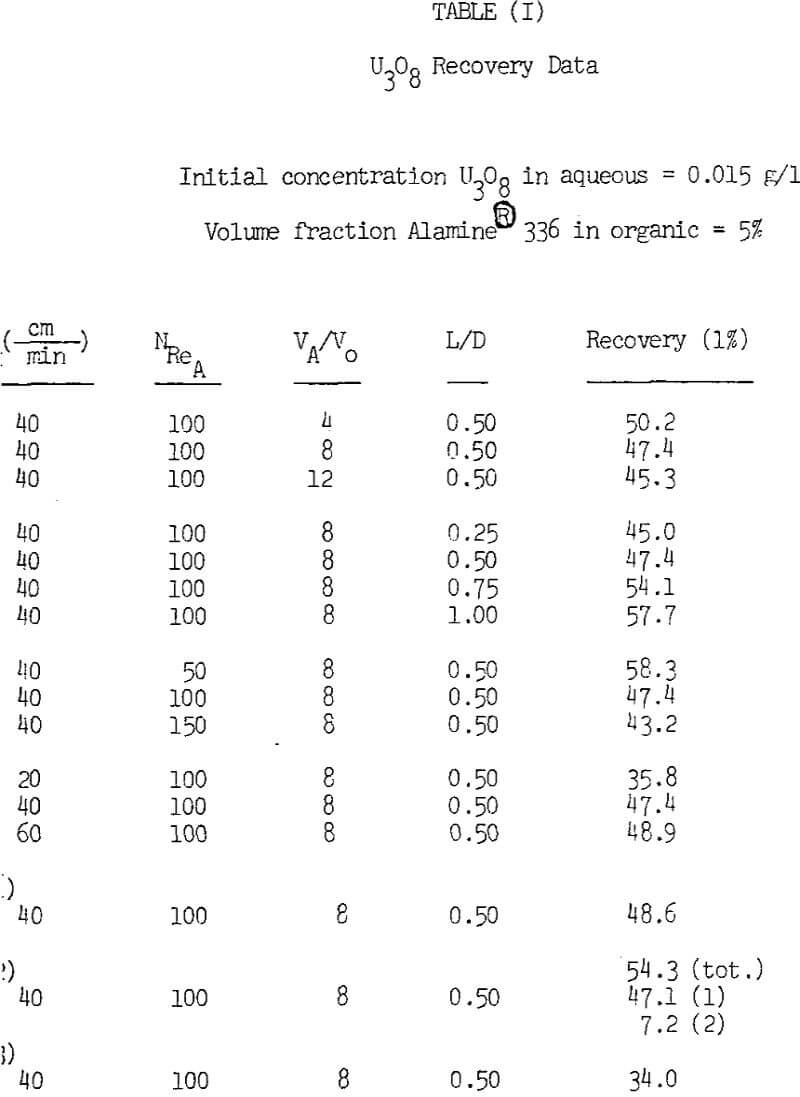
Decreasing the initial concentration of the aqueous feed from 0.015 to 0.010 g/l U3O8 had no effect on uranium recovery. The data also illustrates that operation of two columns in series does not improve extraction significantly. However, recovery was significantly higher for a 5% as compared to a 2% volumetric fraction of Alamine 336 in the organic phase. Although the overall recoveries observed are not particularily high, they should be acceptable for the treatment of solutions such as dump liquors where the metal value is continuously recycled back to the dump.
Uranium recovery decreases slightly when the phase volume ratio in the column is increased. As stated previously, a region of good mixing occurs a short distance above the gas disperser in the column. Since good mixing enhances uranium recovery by increasing the contact area between the phases, it is desirable to have organic present in the region just above the disperser. Increasing the volume ratio of aqueous to organic displaces the organic away from the gas disperser and thus decreases the amount of organic in this region.