Table of Contents
The U.S. Bureau of Mines is investigating surfactants added to water sprays to enhance the control of dust during coal mining operations. The objective of the present work was to establish the general applicability of adding sulfate ion to anionic surfactant solutions in order to improve wetting action. This was pursued through laboratory wettability testing of four anionic surfactants and one nonionic surfactant in combination with various concentrations of sulfate ion.
The experimental results demonstrated that the addition of sulfate ion enhanced the wetting characteristics of all the anionic surfactants when applied to hard-to-wet coals, while easy-to-wet coal showed more complex wetting behavior. Sulfate ion in the presence of nonionic surfactant enhanced wetting only slightly or not at all.
Surface tension measurements of the dilute aqueous surfactant-sulfate anion solutions indicated this property was not important in coal-wetting phenomena, as long as the value was below the critical value for coal. Sulfate ion helps surfactant reduce surface tension but primarily appears to affect wetting action by altering the adsorption characteristics of surfactant on the coal surface.
Coal dust arising from mining creates health and safety problems. Pneumoconiosis (black lung disease) is a serious health problem frequently afflicting coal miners exposed to respirable coal dust for long periods. Also; coal dust suspended in the underground mine atmosphere constitutes an explosion and fire hazard. In the case of methane ignition, suspended coal dust can help propagate fire rapidly throughout a mine to entrap miners before they have a chance to escape. Since 1969, the Coal Mine Health and Safety Act has limited coal mine atmospheres to a maximum of 2.0 mg/m³ of respirable coal dust, or lower if the dust contains >5 pct silica. Mine operators use various methods to control dust levels, including water sprays directed at the face and bit during coal cutting. However, for many mining operations, particularly longwall sections, water spray and other conventional methods are inadequate to reduce levels below the standard threshold limit.
Surfactant addition to the water used in sprays has been suggested and used in some mines for improving the effectiveness of water sprays. A surfactant added to water generally helps the wetting of coal since the coal surface is normally very hydrophobic in nature. Because of the improved wetting action with surfactant, one would predict a favorable effect on coal dust suppression. However, the mine experience using surfactants has not been consistently positive and has been frequently below expectations. Even laboratory testing of surfactant wetting agents has shown numerous inconsistencies among various surfactant products applied to different coals and a lack of correlation with field testing. Accordingly, the U.S. Bureau of Mines is investigating the fundamentals of coal wetting in the belief that a basic understanding of the wetting mechanism is needed to properly evaluate the dust suppression effectiveness of surfactants and to achieve the potential that these reagents appear to offer for reducing coal dust levels in the underground mining environment. This work is in support of the Bureau’s mission to maintain a healthful and safe working environment in the mines by developing technology that enables industry to comply with current regulations in a cost-effective manner.
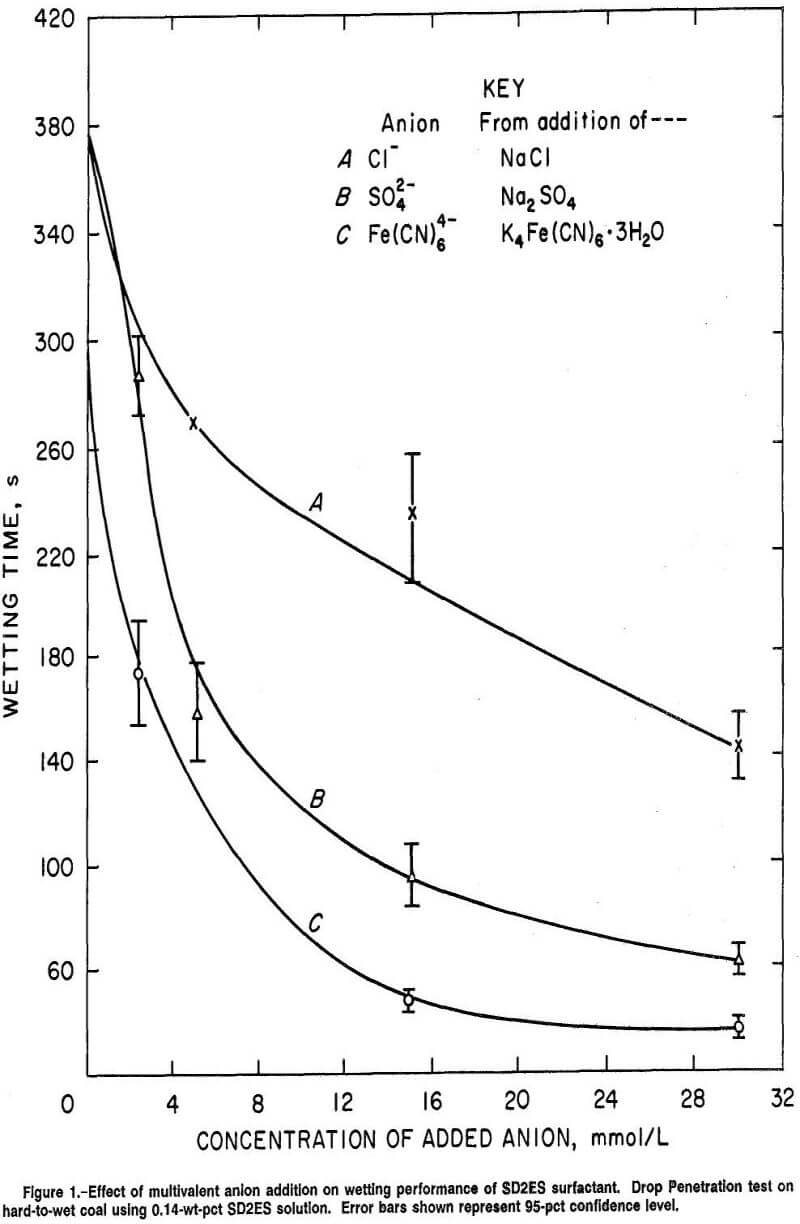
In earlier Bureau work, the coal-wetting ability of sodium di (2-ethylhexyl) sulfosuccinate anionic surfactant (SD2ES) was observed to be greatly enhanced through the addition of sodium and potassium salts of multivalent anions to the surfactant solution. Some of the experimental wettability results for hard-to-wet coal and SD2ES surfactant using the Drop Penetration test are presented in figure 1; Cl-, SO4²-, and Fe(CN)6 4- were the anion species used to promote wetting. The error bars shown in this figure and subsequent figures represent the 95-pct confidence level. It is evident that the wetting improvement (indicated by shorter wetting times) provided by the anion additive was substantial, and that the effect increased as the valence and concentration of the anion were increased. Thus, the percent wetting improvement calculated for SD2ES surfactant plus 30 mmol/L concentrations of Cl-, SO4²-, and Fe(CN)6 4- compared with surfactant alone was 61.9, 83.6, and 90.4 pct, respectively.
The purpose of the present work was to determine if the results in figure 1 are unique to SD2ES surfactant or whether the presence of multivalent anions would improve the wetting of anionic surfactants in general. To prove this point, wettabilities for three other anionic surfactants (besides SD2ES) and a nonionic surfactant were determined in combination with SO4²- anion, from sodium sulfate (Na2SO4), using the Drop Penetration test applied to samples of hard-to-wet coal and easy-to-wet coal.
An easy-to-wet coal is defined as a coal requiring considerably lower concentrations of surfactant to achieve substantial wetting in the Drop Penetration test, compared with hard-to-wet coal. Easy-to-wet coal was also found in earlier work to be capable of readily absorbing pure water by capillary action when the coal powder was packed in a column and contacted with the liquid surface for the Capillary Penetration test. Sulfate ion was chosen as the multivalent anion additive since it gave wetting improvements approaching that of Fe(CN)6 4-, but with the advantage of being a safe and economical reagent for use in practical applications.
The surfactants were chosen on the basis of their ability to wet coal readily, as judged from previous Bureau work (to be published). Surface tensions of the wetting solutions were also measured to determine the effect this factor has on coal wettability and to guide the derivation of hypotheses to explain the observed experimental phenomena.
There are several factors that can influence the wetting action of surfactant solutions applied to the surface of coals. These factors include the surface tension of the liquid solution, the adsorption of surfactants and ions from, the solution onto the coal surface, the exchange of surfactant ions with coal surface ions, the packing of surfactant molecules on the surface, and the adsorption of surfactant on previously adsorbed surfactant to form agglomerates called hemimicelles. A theoretical description of these processes is provided in the following section.
Theory
Critical Surface Tension
Pure water is normally ineffective in wetting coal because of its high surface tension of 72.8 dyn/cm. A considerable reduction in this surface tension is required for successful coal wetting. A critical surface tension was determined by Parekh and Aplan to be about 45 dyn/cm for all coals, independent of rank. Once this critical value is attained, it is believed that other factors, particularly surfactant adsorption, begin to be important and dominate the wetting action.
All the initial surfactant concentrations used in this work were adequate to provide sufficient reduced surface tension for wetting of coal. However, it is possible that for the smallest initial concentrations, adsorption of surfactant on the coal may consume the reagent in the droplet to a point where the concentration is borderline for maintenance of adequate reduced surface tension in the final stages of coal wetting in the Drop Penetration test.
Surfactant Adsorption
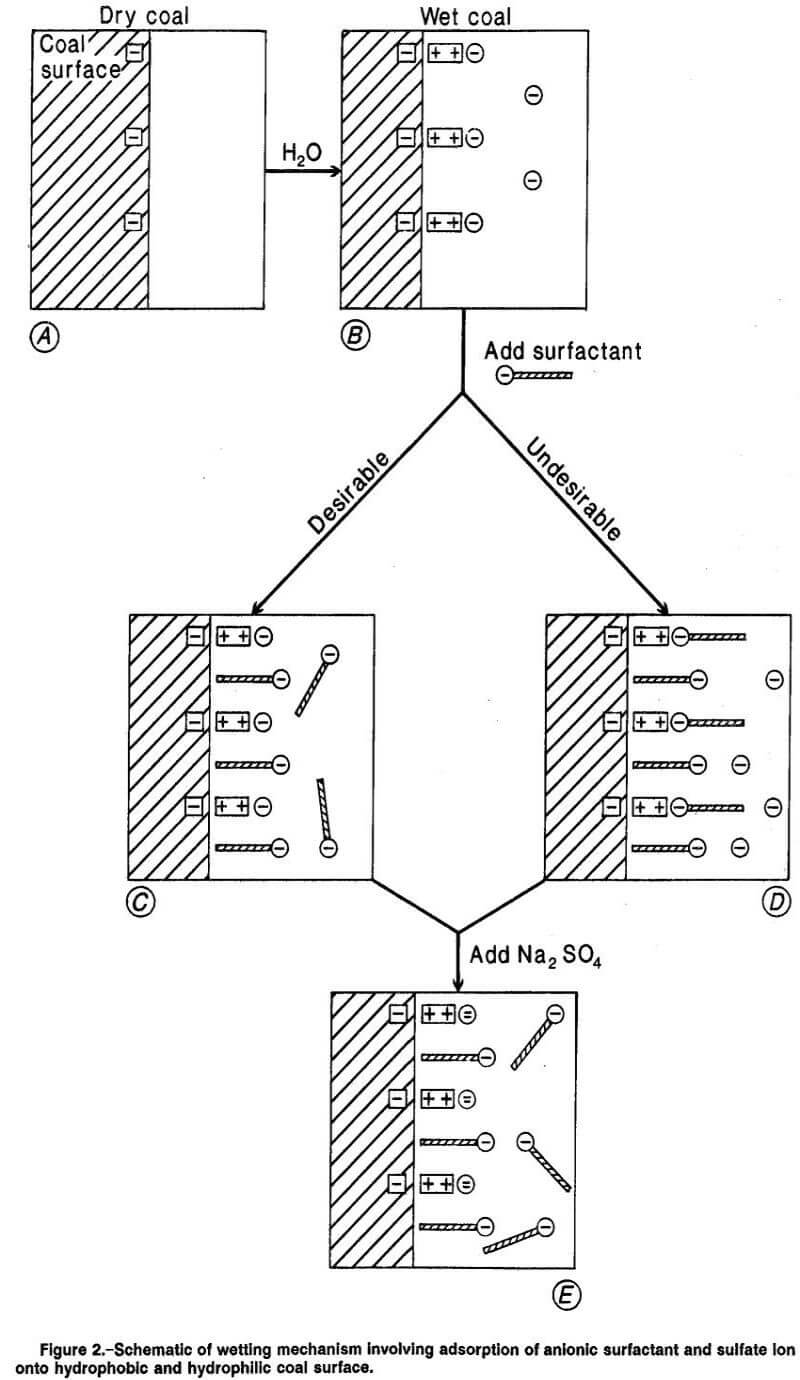
In addition to reducing surface tension below the critical value, the object of adding surfactants is to convert the hydrophobic areas of the coal surface to a hydrophilic state by the adsorption of the surfactant exclusively on hydrophobic sites. If this condition is accomplished, then improved wettability of the coal surface will result (if the liquid surface tension is below the critical value). The coal surface is conceived as composed of both hydrophobic and hydrophilic sites. The adsorption of surfactants on these different kinds of sites complicates the wetting behavior of coal in surfactant solutions. The situation is depicted schematically in figure 2, where the hydrophobic coal surface is shown as the shaded area and hydrophilic sites as small squares enclosing a negative charge (box A). The negatively charged hydrophilic sites stem from oxidation of organic surface groups and the presence of inorganic oxide impurities.
It is suggested that inherent moisture in the coal provides a medium for solubilization of multivalent positive ions from the coal. These cations are attracted to the negative sites on the coal surface to form a layer of positive ions on hydrophilic sites as illustrated (box B, figure 2). The moisture content of the coal, by providing a medium for diffusion through the coal, may determine the extent of the distribution of the positive ions on the sites. Also, development of additional negative sites through oxidation upon exposure to air and distribution of positive ions through an adsorbed moisture layer after exposure may occur.
To achieve improved wetting of coal, through application of surfactant, the desirable objective is for the hydrocarbon, hydrophobic tail of the surfactant to attach itself to the hydrophobic areas of the coal surface. This attachment is the result of van der Waals’ forces between hydrocarbon molecules of the coal surface and the hydrocarbon portion of the surfactant molecule. This adsorption orients the surfactant molecule with its hydrophilic head toward the aqueous phase, as shown in figure 2 (box C). It is to be noted that the figure is an approximate representation. The tails of the surfactant molecules are proportionately much longer than depicted, and therefore, any repulsion forces between surfactant head and nearby hydrophilic coal sites are minimal. This orientation effectively converts the hydrophobic coal sites to a hydrophilic or wettable state since the hydrophilic portion of the adsorbed surfactant molecule is directed toward the aqueous phase. The negatively charged, hydrophilic wetting sites on the coal surface, in this case, remain unchanged as far as ease of wettability is concerned.
Ion Exchange
The hydrophilic head of the surfactant molecule is also capable of being attracted to the positive layer of ions on the coal’s natural hydrophilic sites. In the case of anionic surfactants, this attraction is likely to be especially strong because of the electrostatic forces between the negatively charged head of the surfactant ion and the positively charged ion layer on the coal surface. This attraction results in the displacement of monovalent negative ions previously attached to the positive ion layer in an ion-exchange type of reaction.
This ion-exchange reaction, where negative surfactant anions replace other attached anions on the positive ion layer, converts the hydrophilic sites to an undesirable nonwetting state because of the orientation of the attached- surfactant with its hydrophobic tail directed toward the aqueous phase (box D, figure 2). The addition of Na2SO4 provides divalent sulfate anions that compete with mono-valent surfactant anions for attachment to the layer of positive ions on the coal surface. Because of their higher valence charge compared with that of surfactant anions, sulfate anions will tend to be preferentially adsorbed on the positive ion layer, displacing surfactant anions following the affinity hierarchy of ion-exchange reactions. This mechanism prevents conversion of these hydrophilic sites to a hydrophobic state with the attendant decreased wettability (figure 2, box E).
The affinity hierarchy may not prevail if an excess of monovalent surfactant ion species over divalent sulfate species is present in solution. In such a case, the protection of hydrophilic coal sites afforded by sulfate anion would be diminished. An analogy from water treatment technology is the process of regenerating an ion-exchange resin by using high concentrations of Na+ ions (from NaCl brine) to remove attached higher valence Ca+2 and Mg+2 ions from the resin. It is to be noted that nonionic surfactants would be unaffected by ion-exchange effects. Thus, nonionic surfactants, if adsorbed on the positive ion layer of the coal, would not necessarily be removed or affected by addition of Na2SO4. Also, electrostatic repulsive forces would be ineffective to prevent adsorption of nonionic surfactant on top of previously attached sulfate ions.
Close-Packing Effects
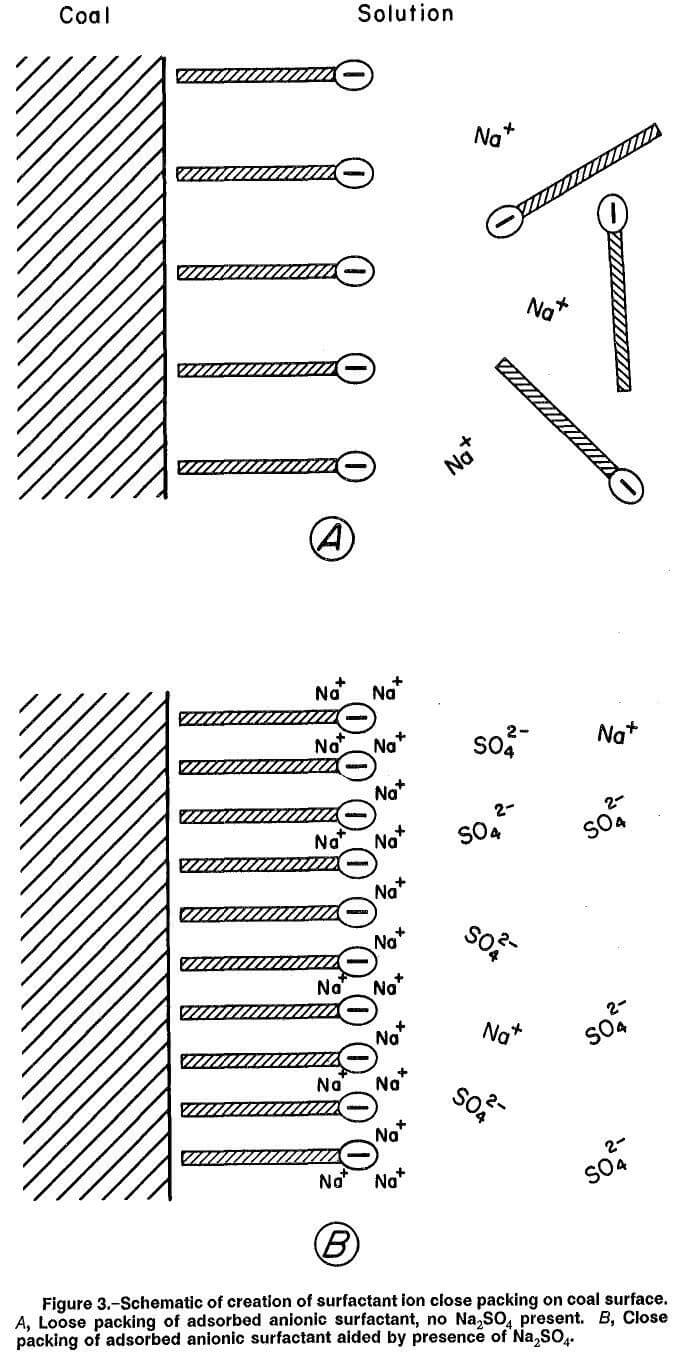
The addition of Na2SO4 electrolyte provides numerous ions (Na+ and SO4²-), which can reduce the distance over which electrostatic forces in the double layer are effective; i.e., the Debye length is shortened. In effect, the “cloud” of ions from the electrolyte can screen or reduce the repulsive electrostatic forces between the negatively charged heads of the anionic surfactant adsorbed on the hydrophobic coal surfaces. As a result, greater density of the adsorbed surfactant ions is permitted, which tends to convert more of the coal’s hydrophobic surface to a hydrophilic condition and increase wettability. This situation is depicted in an approximate manner in figure 3. Part A of the figure shows the low-density adsorption of surfactant ions on the coal surface occurring in the absence of Na2SO4. Part B shows the increased density of surfactant adsorption occurring in the presence of Na2SO4, caused by the reduction of repulsive forces between the negatively charged heads of the adsorbed surfactant ions.
Similar improved close packing of anionic surfactant molecules through Na2SO4 addition can also occur at the air-liquid interface in aqueous solutions of surfactant. This phenomenon reduces the liquid surface tension of anionic surfactant solutions with increasing concentrations of Na2SO4.
Hemimicelle Formation
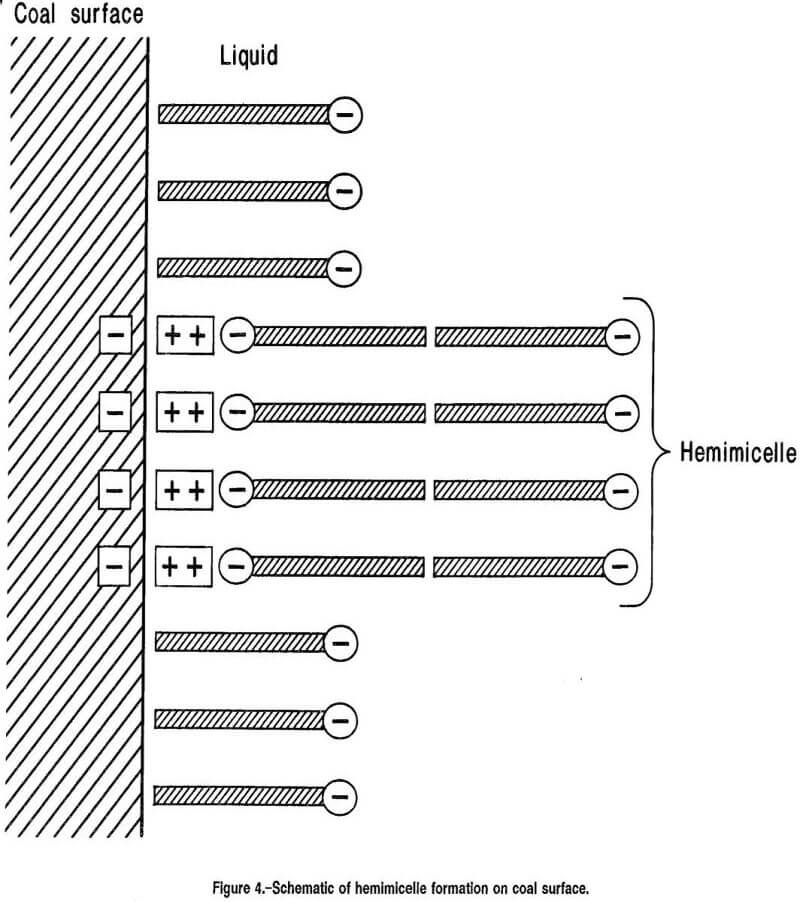
An additional factor in the adsorption of surfactant molecules is the possibility of hemimicelle formation on the coal surface. Adsorption of anionic surfactant on positively charged surfaces is believed to occur in three distinct modes as surfactant concentration is increased. At low concentration, surfactant adsorbs mainly by ion exchange. At intermediate concentrations, a marked increase in adsorption occurs resulting from interaction of the hydrophobic chains of oncoming surfactant anions with those of previously adsorbed surfactant and with themselves. This aggregation of the hydrophobic groups of the surfactant has been called hemimicelle formation. An example of hemimicelle formation on a cluster of hydrophilic sites on the coal surface is depicted schematically in figure 4. A cluster of surfactant ions is shown attached to a group of adsorbed dipositive cations on the coal surface. The hydrophobic tails of these surfactant molecules extending into the solution are capable of attracting further surfactant anions to form the aggregation depicted in the figure. The effect of this kind of hemimicelle formation is to restore wettability to the coal surface lost by the first layer adsorption of surfactant in adverse orientation. The final mode of adsorption, labeled the “electrostatically hindered mode”, is weak because further surfactant adsorption must overcome electrostatic repulsion between oncoming surfactant ions and similarly charged hemimicelles on the coal surface.
Materials
Coals
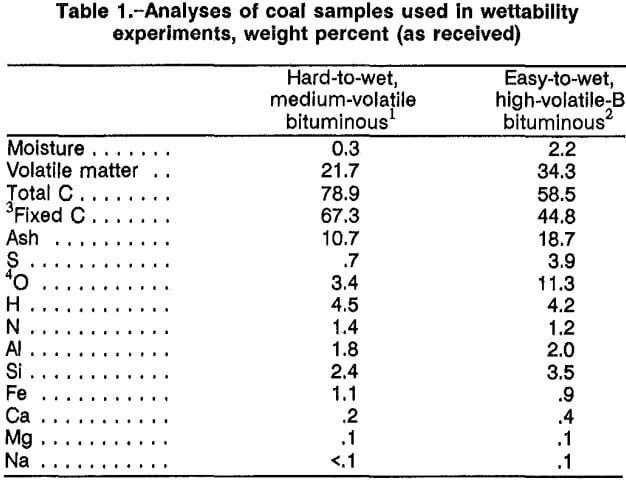
Chemical analyses of the hard-to-wet and easy-to-wet coals tested are given in table 1. The apparent ranks of the coal samples were based on ASTM D388-84 classification system. The two samples were received in the form of 3-in lumps and were ground to minus 200 mesh for the Drop Penetration test. The grinding was done in air, but care was taken not to overgrind the samples so as to lessen oxidation of the coal surfaces. Laser light- scattering measurements of the ground particles showed 74 pct of the hard-to-wet coal and 69 pct of the easy-to-wet coal to be in the size range of 11 to 62 µm. The two coals chosen were selected on the basis of their extreme wetting properties, the one being difficult to wet and the other wetting easily at low concentrations of surfactant in the Drop Penetration test.
Wetting Solution Reagents
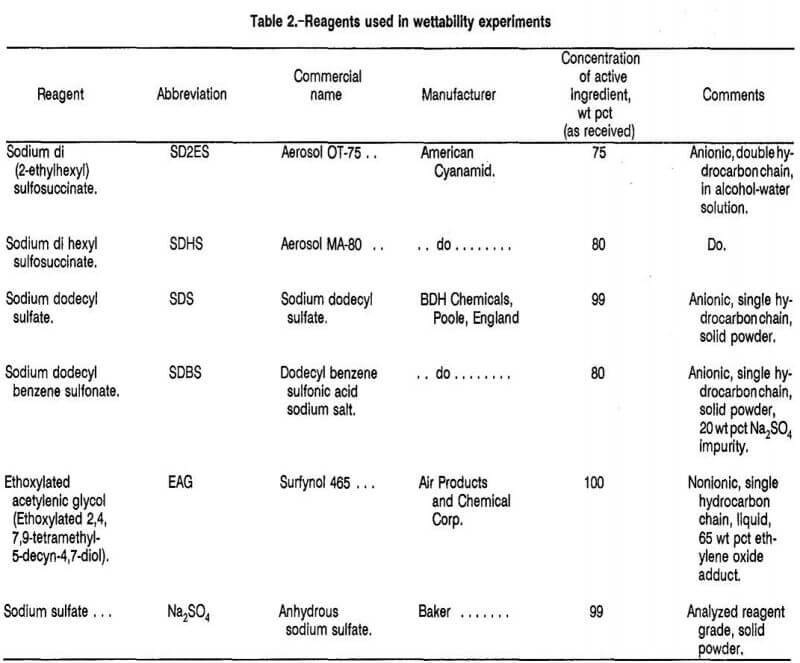
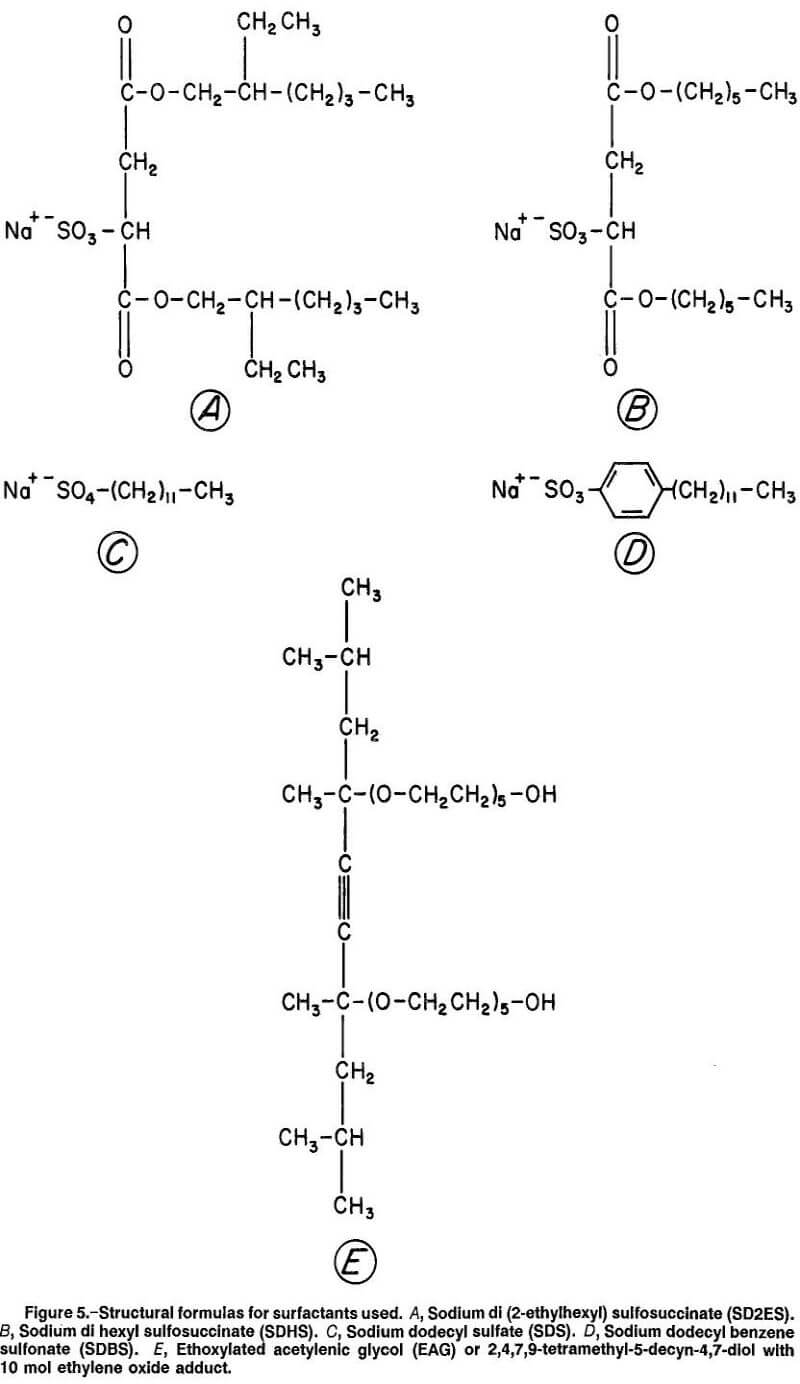
Four of the surfactants tested were anionic; i.e., they formed negative anions when dissolved in water. These surfactants were compared with a nonionic surfactant with and without electrolyte (Na2SO4) addition. Table 2 lists the surfactants and electrolyte reagent used in the wettability testing. The structural formulas for the surfactants are given in figure 5.
The wetting solutions were prepared by diluting the surfactant reagents to the desired concentration with pure water to obtain a 3-L quantity of solution. This solution was then divided into 500-mL aliquots in volumetric flasks. Na2SO4 was added to the aliquots to achieve the desired concentrations of added SO4²- ion (usually 2.5, 5.0, 15,0, and 30.0 mmol/L). It is to be noted that all the surfactant concentrations given for the wetting solutions in this report are expressed in terms of active surfactant ingredient. The pure water used to prepare the surfactant wetting solutions was first distilled and then passed through a deionizer cartridge.
Experimental Procedure
The extent of wetting achieved with the various wetting solutions, when applied to the two wettability classes of coal, was measured with the Drop Penetration test. This test was described in earlier work. In this test, a 40-µL droplet of the surfactant solution to be tested is deposited (from a micropipette) onto the planar surface of a bed of minus 200-mesh coal particles. The droplet is observed through a microscope (x20), and the time for the droplet to completely fill with coal particles is recorded with a stopwatch. The coal bed was 8 mm in depth, and its top surface was smoothed and leveled with a straight-edge, avoiding any packing. Determining the point of complete filling of the droplet was difficult because some wetting solutions formed thin liquid films near the end-point, which remained long after the droplet was apparently filled with particles. It was observed that as the particles filled the droplet, a stage was reached in which only a small amount of liquid was visible, flowing in small rivulets on the surface of the filled droplet. Consistency in determining the endpoint was attained by designating complete particle filling as the point at which no coal particle movements could be observed in any of the rivulets (believed to correspond to the cessation of particle penetration into the droplet, resulting in the stopping of fluid flow in the rivulets). Generally, the Drop Penetration test was repeated 10 times for a wetting solution on a coal sample, and the average of the trials was computed. The standard deviation in the computed average time for complete particle filling was typically 10 to 15 pct. No attempt was made to test very dilute solutions of wetting agents where wetting times exceeded 12 min because of the experimental inconvenience and because evaporation of the liquid droplet might become a factor.
Surface tensions of all wetting solutions at 28° C were also measured with a du Nouy ring-type instrument. The particle size distributions of the ground coal samples were measured with a Microtrac Particle-Size Monitor, model 7981, Leeds and Northrup Co.
Experimental Results
Pure Water
No wetting action was observed in the Drop Penetration test on either coal sample by pure water. This expected result is a consequence of the high surface tension of water, which must be lowered below the critical value before coal wetting can proceed.
SD2ES Surfactant on Hard-to-Wet Coal
For SD2ES surfactant solutions without sulfate additive, applied to the hard-to-wet coal sample, the wettability increased as the surfactant concentration was increased. A wetting time limit was reached at about 10 s for 0.61 wt pct SD2ES concentration (fig. 6), at zero concentration of added Na2SO4. Increasing the SD2ES concentration to 0.82 wt pct did not significantly lower the wetting time at zero concentration of added Na2SO4. In general, it was found in this and other work that 7 to 10 s wetting time is a minimum value in the Drop Penetration test (when employed as described) regardless of the wetting agent or coal employed.
Certain characteristics visible in figure 6 reveal the dominant influence of adsorption phenomena on the wetting action at the coal-liquid interface compared with the influence of the liquid’s bulk properties. Figure 6 also shows remarkable improvement in wetting time with only a slight increase in SD2ES concentration from 0.14 to 0.20 wt pct at zero concentration of added Na2SO4. This wetting response suggests that adsorption of surfactant on hydrophilic coal sites is a major factor contributing to the wetting behavior. This is inferred from the almost negligible difference in liquid surface tensions of these two wetting solutions. The small difference in the two measured values (28.4 dyn/cm for 0.14 wt pct SD2ES and 28.0 dyn/cm for 0.20 wt pct SD2ES) indicates a lack of surfactant molecule close-packing change at the air-liquid interface, which probably reflects a similar situation at the coal-liquid interface. Further improvements in wettability occurred with further increased SD2ES concentration but with increasingly less effect, up to the wettability limit at 0.61 wt pct SD2ES (curves C and D).
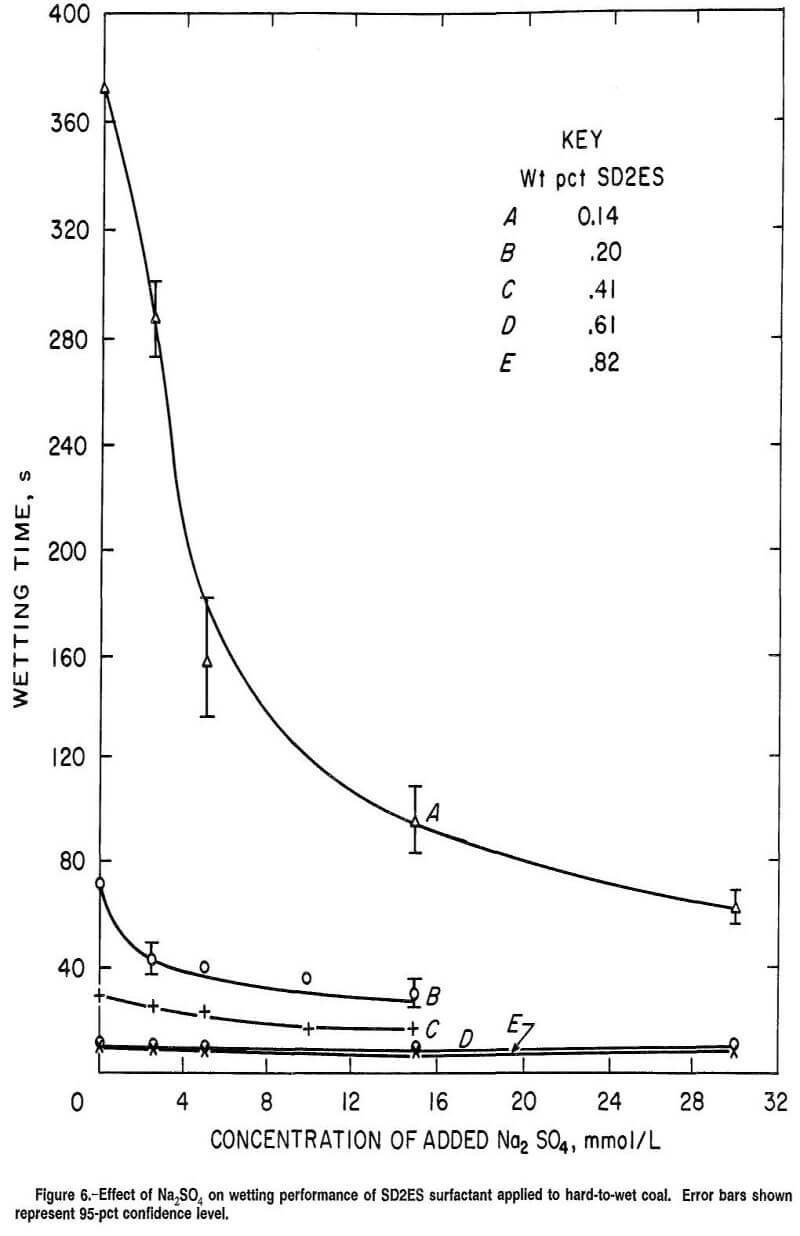
Hard-to-wet coal is conceived as having a preponderance of hydrophobic surfaces and a smaller fraction of hydrophilic sites, compared with easy-to-wet coal. It is suggested that adsorption of surfactant (in the absence of Na2SO4) occurs simultaneously on hydrophobic and hydrophilic surfaces at low surfactant concentrations. For low concentrations, adsorption of surfactant on hydrophilic coal sites would have a significant negative effect on wettability since not only are hydrophilic coal sites converted to a hydrophobic state, but scarce surfactant anions are consumed in the process, leaving less reagent available for desirable adsorption on hydrophobic sites to improve the total coal wettability (figure 2, box D). Although simultaneous adsorption occurs, the attraction of surfactant to hydrophobic sites is believed somewhat greater than to hydrophilic sites. This results in a net wetting improvement as surfactant concentration is increased, as seen in figure 6. For hard-to-wet coal, a point is reached at which adsorption on all available hydrophilic sites has reached saturation, so that any further adsorption occurs solely on the remaining hydrophobic sites, which constitute the majority of available adsorption sites for hard-to-wet coal.
It is suggested this saturation point in the adsorption of surfactant onto hydrophilic coal sites is reached between 0.14 and 0.20 wt pct SD2ES. The great change in wetting time between these concentrations is believed due to the transition from competing, simultaneous adsorption on the two kinds of sites (with its canceling effect on wettability) to a stage of exclusive, noncompetitive adsorption on hydrophobic coal sites. When the wetting solution is at or below 0.14 wt pct SD2ES, wetting improvement by van der Waals’ adsorption on hydrophobic surface is offset partially by concurrent adsorption of surfactant ions on hydrophilic sites (by electrostatic forces) in opposite orientation to produce hydrophobicity as shown in figure 2 (box D).
At concentrations of 0.20 to 0.61 wt pct SD2ES, adsorption can occur exclusively on hydrophobic sites in the later stages of wetting, resulting in greatly reduced total wetting time. At higher concentrations of surfactant, the wetting improvement change as a function of surfactant concentration is ultimately limited. This may be because a monolayer of adsorbed surfactant has been achieved or because kinetics factors (such as diffusion of particles across the coal-liquid interface) control the adsorption at faster wetting times.
There is the possibility of hemimicelle formation on the coal surface in the later stages of surfactant adsorption, which could also contribute to the sharp drop in wetting time between curves A and B (fig. 6). In this case, after saturation of hydrophilic sites with surfactant, hemimicelle formation occurs atop the surfactant layer to restore hydrophilicity, as in figure 4. This occurrence, together with the attendant conversion of hydrophobic coal surface to a wettable state from van der Waals’ adsorption, could further contribute to the enhanced wetting improvement observed between 0.14 and 0.20 wt pct SD2ES surfactant solutions in figure 6.
As shown in figure 2 (box E), addition of Na2SO4 protects hydrophilic sites from conversion to hydrophobicity by preventing surfactant adsorption in adverse orientation. Figure 6 shows that the greatest improvement from addition of Na2SO4 occurred for a low concentration of surfactant (curved). At this SD2ES concentration (which is just below the transition concentration where hydrophilic coal sites are believed to become nearly or completely covered with adsorbed surfactant), the Na2SO4 addition acts to preserve hydrophilic sites and reduces the number of sites available for surfactant adsorption in adverse orientation. In effect then, Na2SO4 lowers the transition concentration for SD2ES, so that the wetting improvement through Na2SO4 additions is nearly as beneficial as that when SD2ES concentration alone was increased (curved). This occurs because scarce surfactant anions are not only prevented from adsorbing improperly (eliminating hydrophilic surface), but surfactant also then becomes available for adsorption on hydrophobic sites and possibly for hemimicelle formation on any remaining unprotected hydrophilic sites. Fast wetting times can be achieved with high concentrations of surfactant alone as well as with lower concentrations of surfactant in combination with Na2SO4 reagent. Practically speaking, however, it is preferable from an economic standpoint to add inexpensive Na2SO4 reagent to low concentrations of more costly surfactant to improve wetting rather than to increase surfactant concentration.
It is not certain what caused the lack of improved wetting response when Na2SO4 was added to surfactant solutions of higher concentration (figure 6, curves B, C, and D). It may be that the advantageous valence-controlled affinity hierarchy for ion-exchange adsorption of sulfate ion (on the hydrophilic positive layer of the coal) becomes inoperative at these higher concentrations of surfactant. When valence no longer controls adsorption, surfactant ions, because of their high concentration in solution, remain firmly attached to hydrophilic sites on the coal despite the presence of sulfate anion. Alternatively, if hemimicelle formation has taken place on hydrophilic coal surface at higher surfactant concentrations to restore hydrophilicity, the addition of Na2SO4 is seen to be superfluous and consequently ineffective.
SD2ES Surfactant on Easy-To-Wet Coal
The wettability behavior of SD2ES surfactant and Na2SO4 additive on easy-to-wet coal was different in several respects from their behavior on hard-to-wet coal. Figure 7 displays the wetting times measured on easy-to-wet coal as a function of SD2ES and added Na2SO4 concentrations. As with hard-to-wet coal, increasing the surfactant concentration improved the wetting until a limit was reached (curve D). However, unlike with hard-to-wet coal, further increase of SD2ES concentration did not maintain wetting at the limiting value but instead decreased the wetting, the excess SD2ES concentration apparently harming the wetting action above the limiting concentration (curves E and F).
To understand this wetting behavior for easy-to-wet coal, it is suggested that the coal surface again be envisioned as possessing both hydrophobic and hydrophilic surface sites, but in this case the hydrophilic sites outnumber the hydrophobic sites. For surfactant in the absence of Na2SO4 addition, it is likely that the smaller number of hydrophobic coal sites are almost covered by adsorbed surfactant long before the hydrophilic sites, as surfactant concentration is increased. If sufficient surfactant is available, adsorption proceeds exclusively on hydrophilic sites after hydrophobic surface has reached adsorption saturation. However, this final stage of adsorption begins to create increased hydrophobic character on the coal surface because of adsorption of surfactant on the hydrophilic sites in adverse orientation (figure 2, box D). The point at which the net adsorption on the easy-to-wet coal sample (hydrophobic minus hydrophilic adsorption) begins to favor attachment to hydrophilic surfaces to create hydrophobicity is seen to occur in figure 7 at a concentration of about 0.41 wt pct SD2ES. This transition point is marked by a substantial decrease in wettability (longer wetting time, curve E at zero Na2SO4 concentration). Further increase in SD2ES concentration only results in further deterioration of wettability because of further adverse adsorption on hydrophilic sites to create hydrophobicity (curve F).
The possibility of hemimicelle formation was previously mentioned for hard-to-wet coal, but there appears to be no evidence of such phenomena for easy-to-wet coal in figure 7. The absence of restoration of wettability at high concentrations of surfactant (curve F) leads to the conclusion that hemimicelles are not formed on easy-to-wet coal. Furthermore, one may argue that since hemimicelle formation is a property related to surfactant characteristics, the wetting phenomena observed for hard-to-wet coal that was previously attributed to possible hemimicelle formation is due to other causes. However, considerably more surfactant molecules are required to form hemimicelles on easy-to-wet coal because of the greater hydrophilic surface area and, therefore, greater numbers of adsorbed surfactant molecules in adverse orientation. Hence, greater
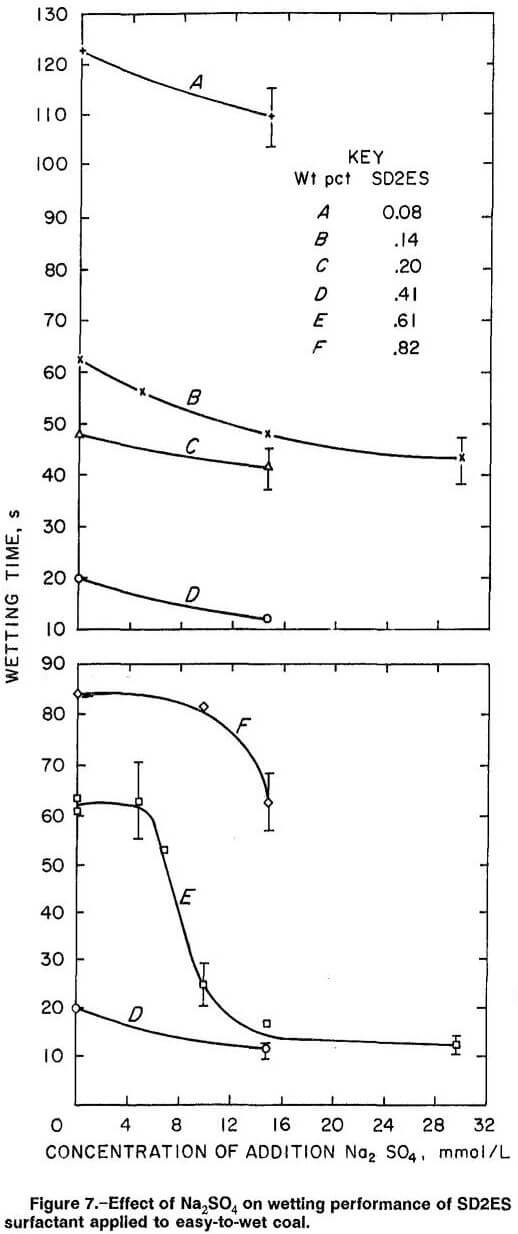
surfactant concentration is required to form hemimicelles on easy-to-wet coal compared with hard-to-wet coal, to offset the hydrophobic effect of adversely adsorbed surfactant. The hemimicelle effect may not be noticeable until concentrations of >0.82 wt pct SD2ES are attained. However, concentrations of >0.82 wt pct SD2ES are not practical because of solubility limitations.
In figure 7 (curves A, B, C, and D), the addition of Na2SO4 generally improved the wettability, but not remarkably except for SD2ES concentrations above the transition concentration for maximum wettability (curves E and F). It is suggested the subdued wetting response at lower surfactant concentration results because much of the sulfate anion adsorbs onto free hydrophilic sites without displacing adsorbed surfactant anions, since surfactant coverage of the numerous hydrophilic sites on easy-to-wet coal is not likely to be extensive at lower concentrations of surfactant. At higher surfactant concentration, above the transition concentration, the addition of Na2SO4 is seen to be much more effective in improving wetting, presumably because the sulfate anion has a greater probability of displacing adsorbed surfactant ions, since the population density of adsorbed surfactant on the hydrophilic surface has probably become substantial at that point. Low concentrations of Na2SO4 (<5.0 mmol/L) appeared ineffective above the surfactant transition concentration (figure 7, curves E and F). This region may indicate there is insufficient sulfate concentration to adequately displace adsorbed surfactant from the hydrophilic surface to give significantly visible wetting effects.
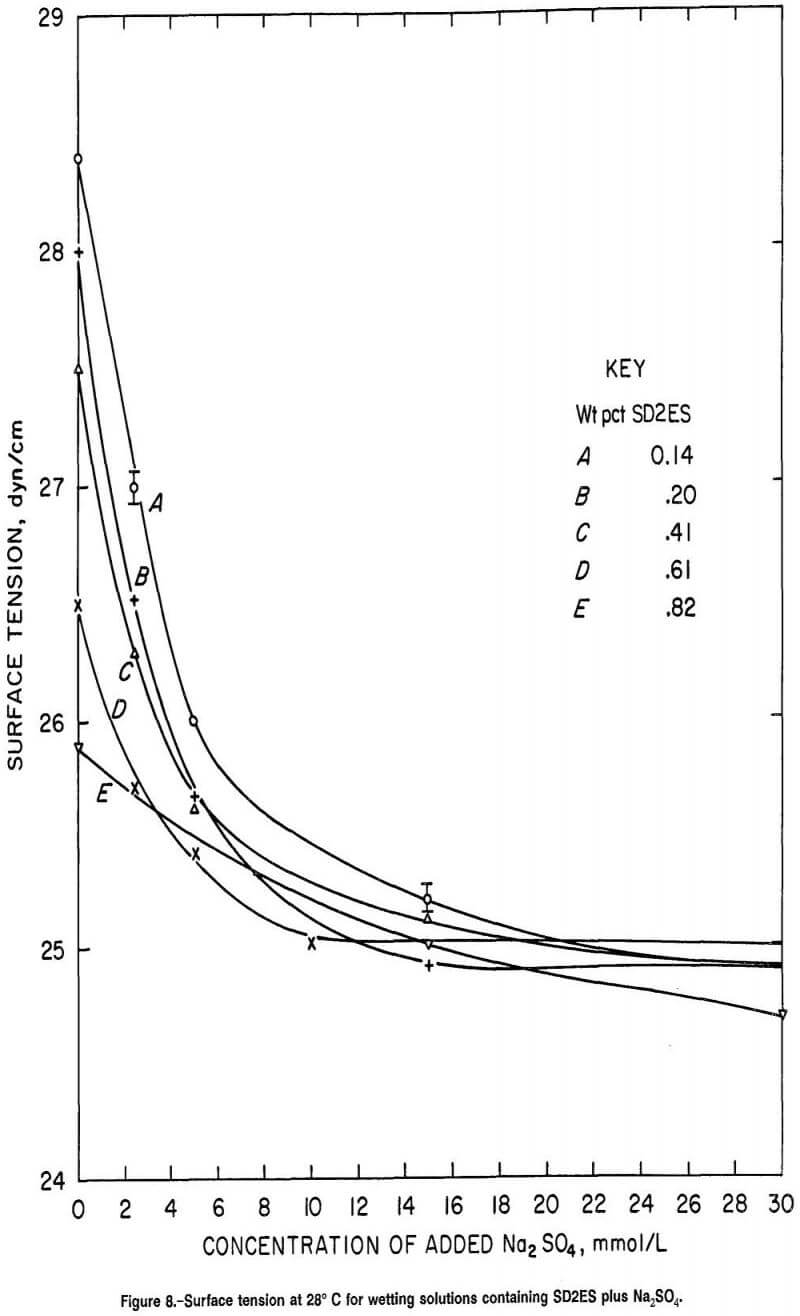
Surface Tension of SD2ES Solutions
Comparison of the surface tension of the surfactant solutions (fig. 8) with their wetting abilities on hard-to-wet coal (fig. 6) reveals few similarities other than as surfactant and Na2SO4 concentrations are increased, wettability tends to increase and surface tension to decrease. However, the large difference in wetting performance between 0.14 and 0.20 wt pct SD2ES is not reflected by a similar large difference in surface tensions between these two concentrations (figure 8, curves A and B). Also, figure 8 shows a consistent and similar decrease in surface tension as Na2SO4 is added to 0.14, 0.20, and 0.41 wt pct SD2ES surfactant solutions, while the wettability improvement from Na2SO4 addition was less effective from dilute to higher concentrations of surfactant (figure 6, curves A, B, and C). For easy-to-wet coal, the impairment of wettability at higher concentrations of surfactant (fig. 7) is not predicted by figure 8, where surface tension declines gradually or remains constant as surfactant concentration is increased.
Other Anionic Surfactants
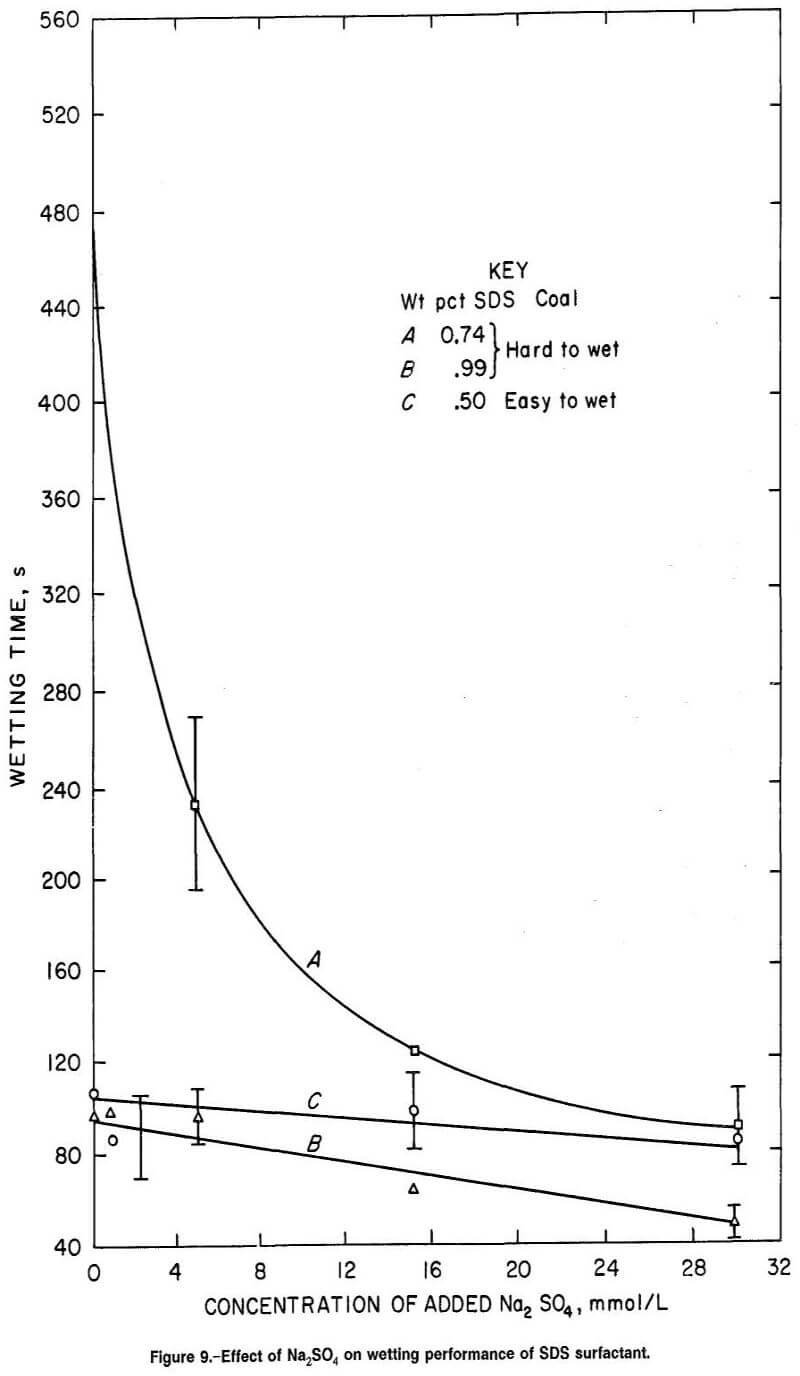
Figure 9 demonstrates that anionic SDS surfactant was effective in wetting hard-to-wet coal, the wetting response increasing as the concentration of the surfactant was increased (curves A and B at zero concentration of added Na2SO4). As with SD2ES, a small increase in surfactant concentration (0.74 to 0.99 wt pct) resulted in a remarkable decrease in wetting time. Again, the addition of Na2SO4 improved the wettability most effectively at the lower concentration of surfactant for hard-to-wet coal (figure 9, A compared with curve B).
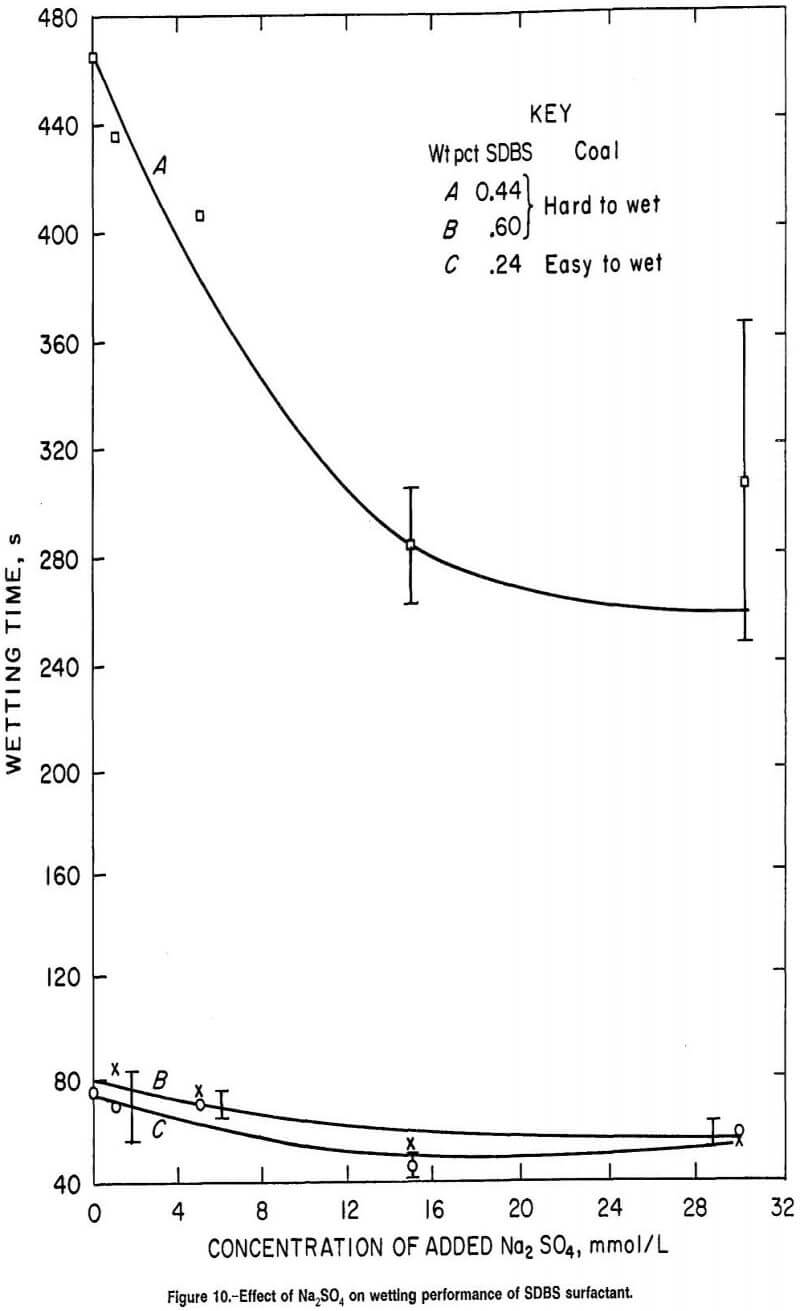
Figure 10 demonstrates the effect of Na2SO4 addition on the Drop Penetration wettability of SDBS surfactant. This surfactant showed wetting characteristics similar to those of SD2ES and SDS on hard-to-wet coals. Greatly improved wetting resulted from small increases in surfactant concentration, similar to the results for SD2ES and SDS (curves A and B). Wetting was improved with Na2SO4 addition at low concentrations of surfactant (curved), but when surfactant concentration was increased, the effect of Na2SO4 addition was small (curve B).
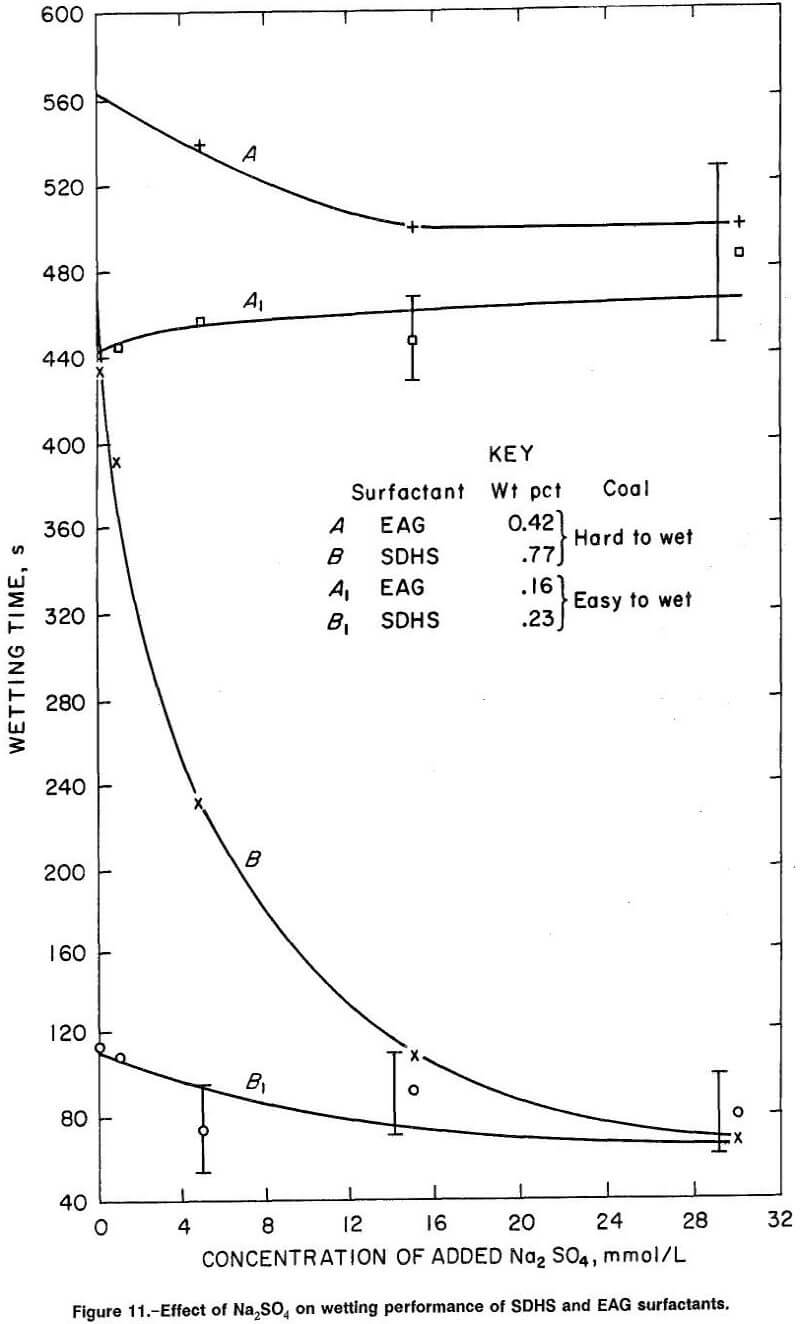
Figure 11 presents the experimental wetting results for anionic surfactant SDHS, curves B and B1 (the figure also includes the nonionic surfactant EAG, curves A and A1 which will be discussed in the next section). The anionic surfactant SDHS demonstrated improved ability to wet hard-to-wet coal as Na2SO4 was added (curve D), a result similar to that for the other anionic surfactants tested in the slow-wetting range (i.e., for surfactant concentrations yielding 300- to 600-s wetting times before Na2SO4 is added).
Thus, two main characteristics of wetting behavior are visible for hard-to-wet coal treated with anionic surfactants and Na2SO4:
- Greatly improved wetting as Na2SO4 is added to surfactant solutions in the slow-wetting range (curve A of figures 6, 9-10, and curve B of figure 11); and
- Diminished effect on wetting as Na2SO4 is added to surfactant solutions in the fast-wetting range, i.e., those solutions that yield, in the absence of Na2SO4, wetting times in the range 10 to 120 s (curves B, C, D, and E, of figure 6, and curve B of figures 9-10).
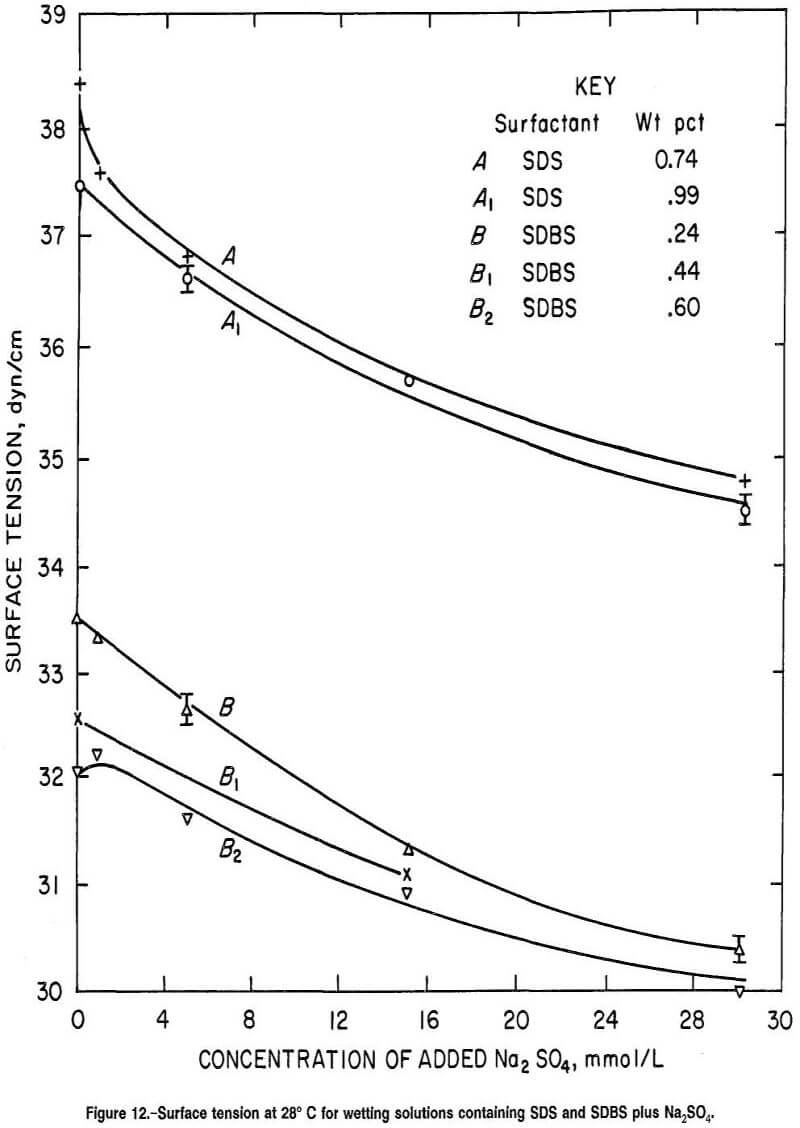
A plot of the surface tension of SDS and SDBS at various concentrations of surfactant and Na2SO4 additive (fig. 12) shows little difference in surface tension between surfactant concentrations, while wetting times can vary widely with concentration (figures 9-10). For example, there is little surface tension difference between curve A and curve A1 of figure 12, while there is a great disparity in wetting times on hard-to-wet coal between these same two concentrations of SDS (figure 9, curves A and B). Similarly, although there is little difference between the surface tension curves for various concentrations of SDBS in figure 12 (curves B, B1 and B2), the wetting times for hard-to-wet coal were greatly different for 0.44 and 0.60 wt pct SDBS (fig. 10).
The effect of added Na2SO4 on SDS, SDBS, and SDHS for easy-to-wet coals (figure 9, curve C; figure 10, curve C; figure 11, curve B1) resembled to some degree the small wetting response displayed by SD2ES on easy-to-wet coal when SD2ES concentration was below the transition concentration (figure 7, curves A, B, C, and D). These anionic surfactants displayed only slight wetting improvement with
increased Na2SO4 concentration, a result in harmony with their evidently weaker adsorbing properties on coal, compared with SD2ES. Thus, it is suggested that there is a smaller number of these weaker absorbing surfactant anions to be displaced from hydrophilic sites by sulfate anion, and once these anions are displaced, adsorption onto hydrophobic sites to produce more wettability proceeds less effectively.
Nonionic Surfactant
For EAG nonionic surfactant applied to hard-to-wet coal (figure 11, curve A), the wetting characteristics are quite different from those of the anionic surfactant. The effect of adding Na2SO4 to this surfactant was minor, with only a small improvement in wetting recorded up to 15 mmol/L Na2SO4 added, and no further improvement at greater concentrations of Na2SO4. For easy-to-wet coal, improved wetting response was completely absent in the presence of Na2SO4 (figure 11, curve A1). This result is expected in light of the proposed wetting mechanisms, since these uncharged surfactant species should not be particularly affected by the presence of ionic species in solution.
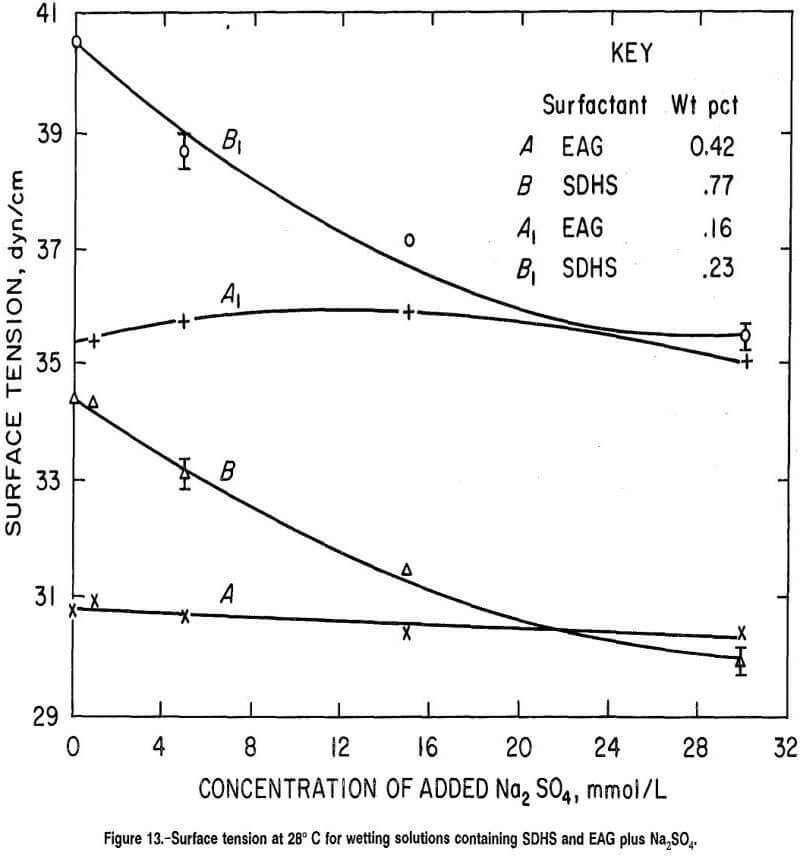
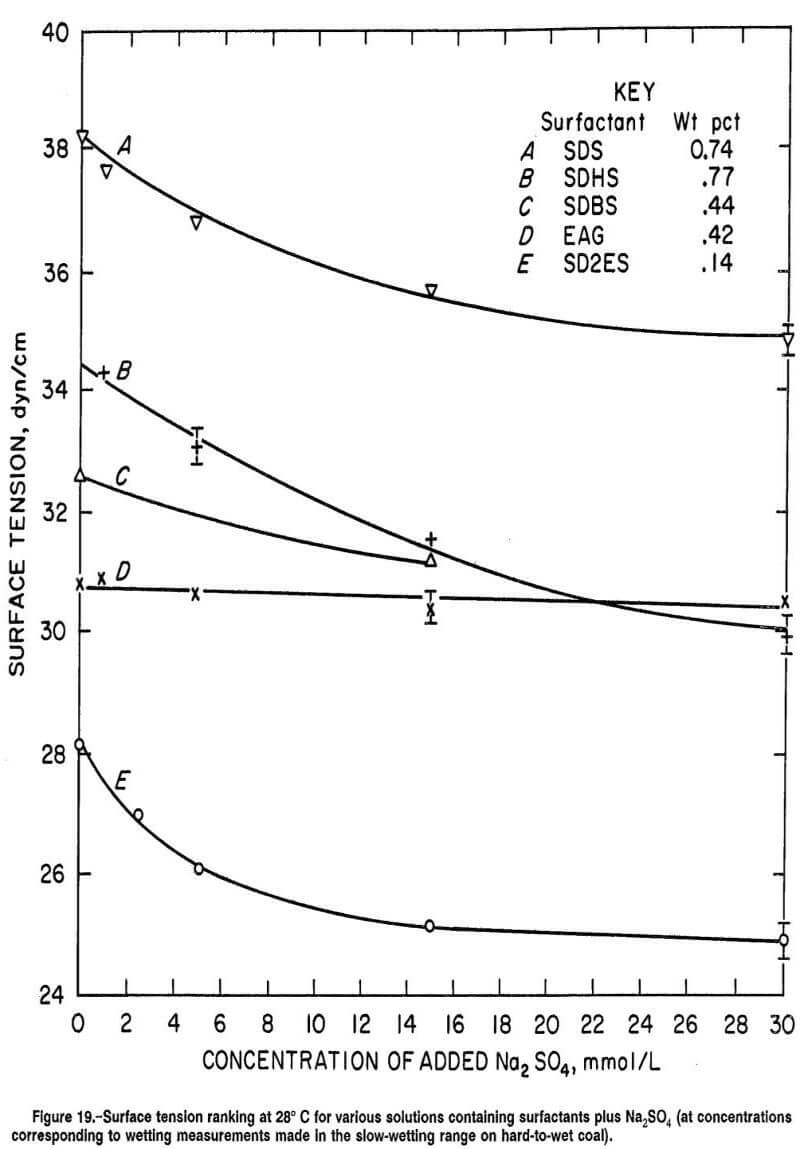
Comparisons of the wetting response for SDHS and EAG surfactant in figure 11 with the surface tension results presented in figure 13 reveal poor correlation between surface tension and coal wettability by these surfactants. It is evident that the class of surfactant (anionic or nonionic) employed in combination with Na2SO4 is much more important than the surface tension reduction afforded by the wetting agent. For example, in figure 13, the surface tensions are almost identical for 0.42-wt pct EAG and 0.77-wt pct SDHS surfactant solutions containing 22 mmol/L of added Na2SO4, while wetting times of the same two solutions differed immensely (by 7 min, curves A and B in fig. 11).
Discussion
Comparison of Surfactant Wetting Performance
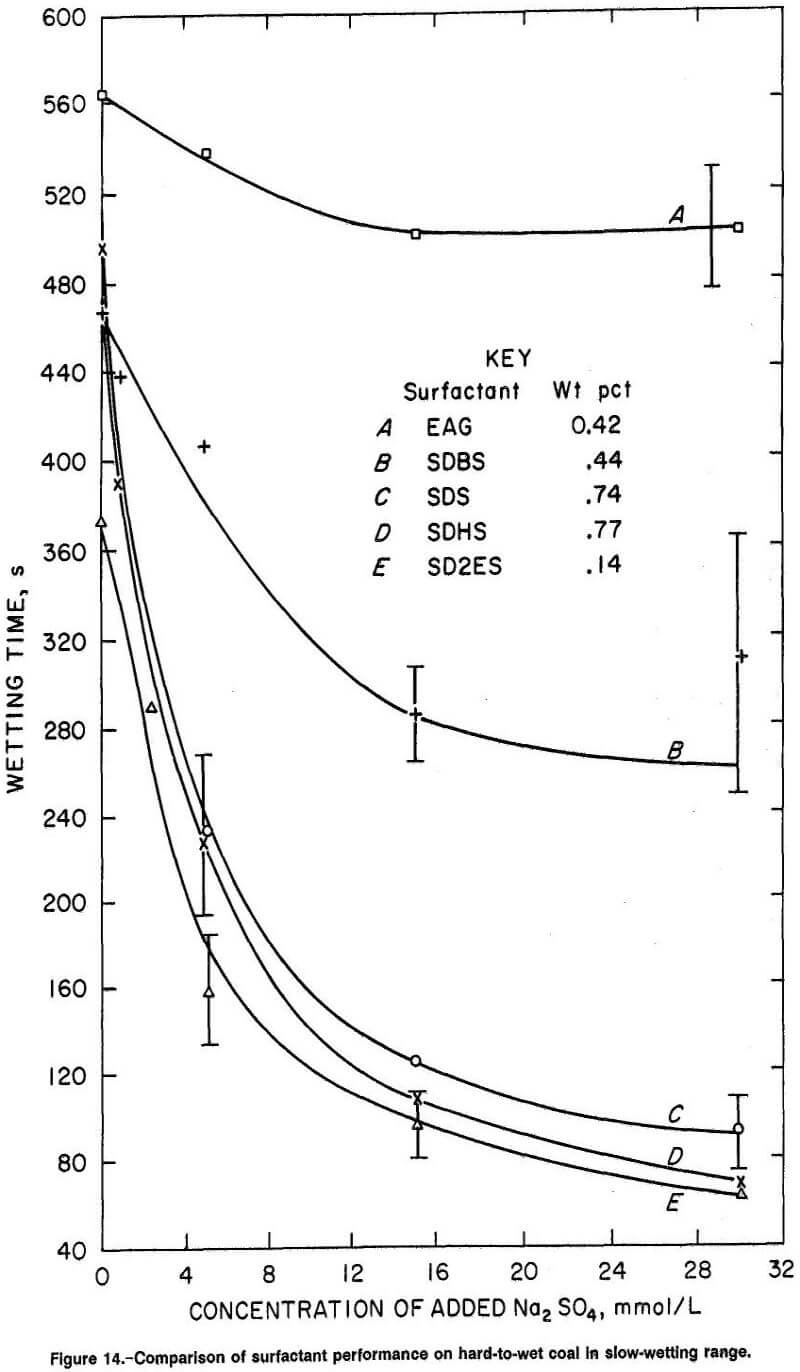
Figure 14 compares wetting curves obtained for the five surfactants on hard-to-wet coal in the slow-wetting range. It is evident that SD2ES is the most effective wetting agent (per unit weight of active ingredient) of all the surfactants tested. SD2ES was observed to have shorter wetting time at 0.14 wt pct concentration than the other surfactants at much higher concentrations. Apparently SD2ES has a greater affinity for hydrophobic coal sites and possibly greater ability to close-pack molecules on the coal surface than the other reagents. This wetting superiority extended also to the observed wettabilities obtained after Na2SO4 addition. The response of SD2ES to the electrolyte addition was sufficient to maintain faster wetting times over the other wetting agents. However, the response of SDS and SDHS surfactants to Na2SO4 addition was particularly strong, enabling these reagents to perform nearly as well as SD2ES in this wetting range, although much higher concentrations of surfactant were required. The response of SDBS to Na2SO4 addition was weaker than that of the other anionic surfactants, which may be related to excess Na2SO4 concentration in SDBS solution resulting from the additional Na2SO4 supplied by the surfactant’s impurity content. The poorest wetting response resulting from Na2SO4 addition was observed for EAG surfactant, a consequence of its nonionic properties.
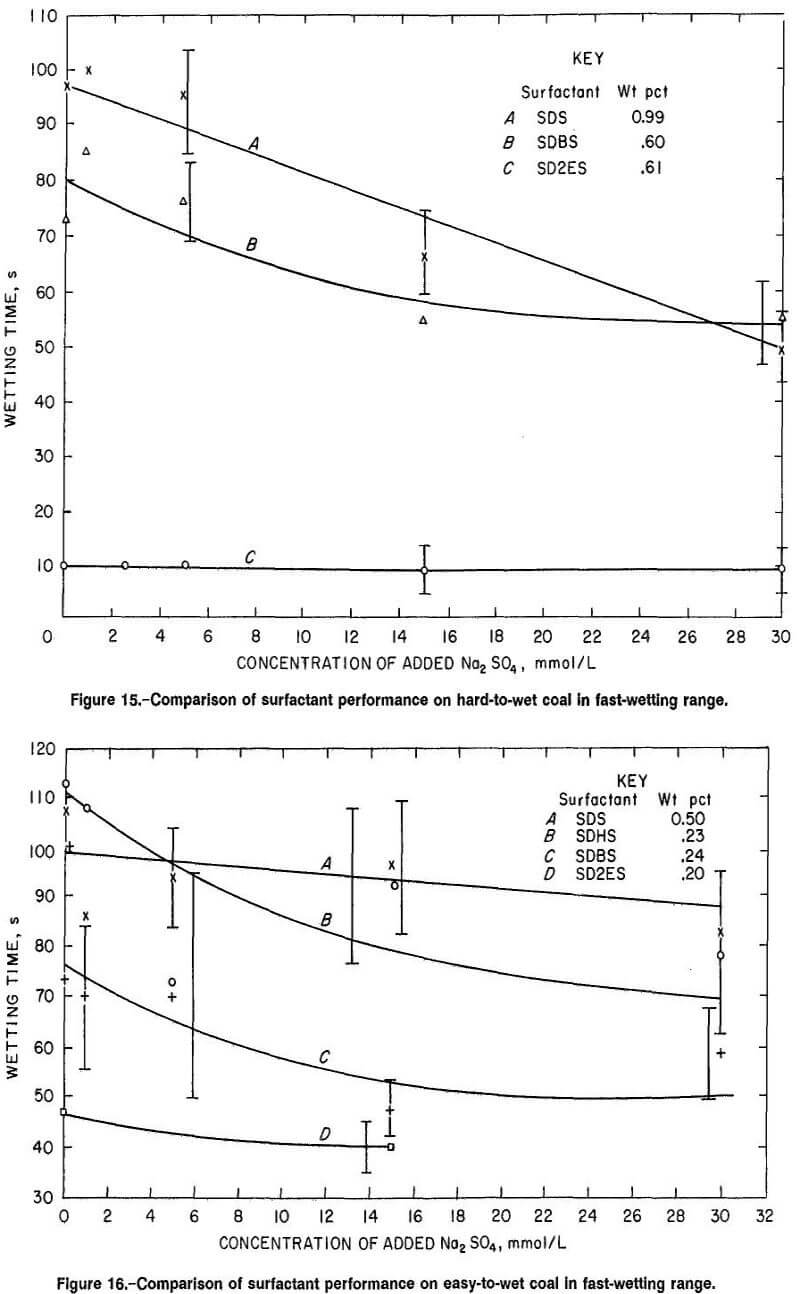
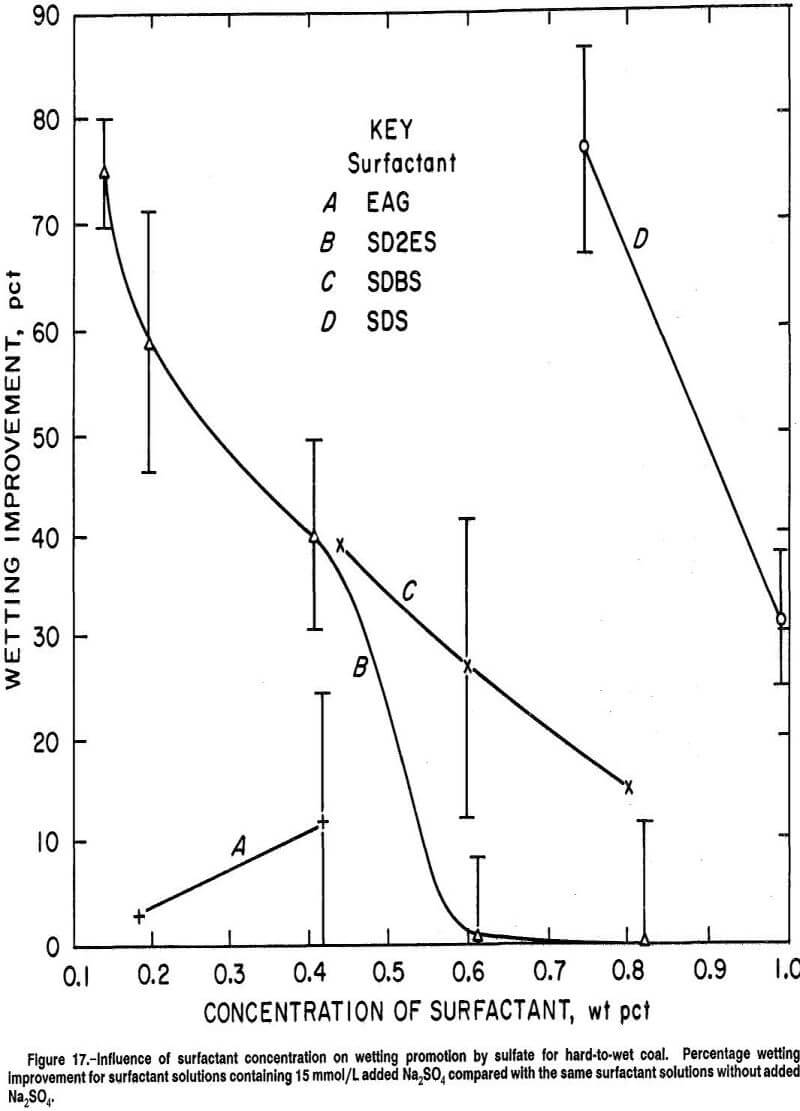
The coal wetting superiority of SD2ES on hard-to-wet coal was also evident for surfactant solution concentrations in the fast-wetting range, but to a lesser degree (fig. 15). For easy-to-wet coal, the wetting responses were rather similar among the anionic surfactants, although SD2ES was still slightly more effective (fig. 16). In the wetting range shown, Na2SO4 addition was not particularly effective for any of the surfactants.
Effect of Surfactant Concentration on Wetting by Sulfate
Hard-to-Wet Coal
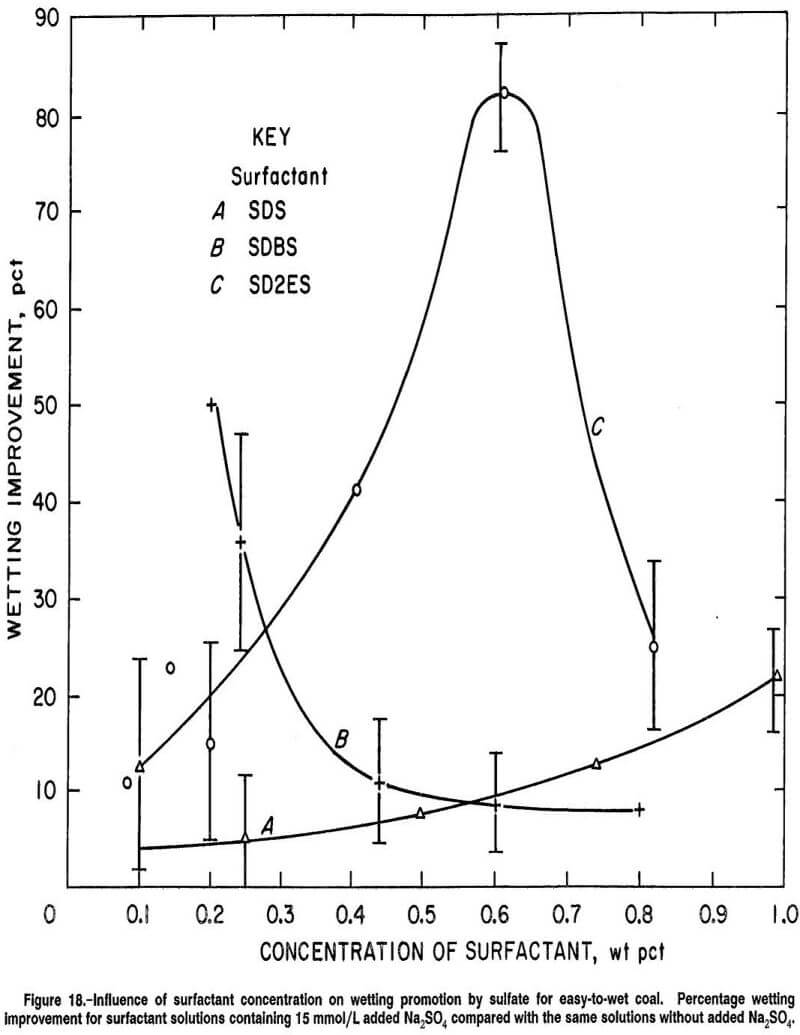
It was observed earlier that the wetting response of anionic surfactant solutions to Na2SO4 addition is greatly dependent on surfactant concentration. Figure 17 compares this concentration dependency for three anionic surfactants (SD2ES, SDBS, and SDS) and the nonionic EAG surfactant when applied to hard-to-wet coal in the presence of 15 mmol/L added Na2SO4. The ordinate in the figure is given in percentage units of wetting improvement (as defined earlier) to compress the scale for better ease of comparison. Generally, it is seen from the figure that the promotion of wetting from Na2SO4 addition (on hard-to-wet coal) was decreased for anionic surfactants as the surfactant concentration was increased. On the other hand, changing the concentration of EAG surfactant had only a small effect on promotion of wetting by Na2SO4.
SDBS surfactant demonstrated less response to concentration changes than SD2ES and SDS when compared in the concentration ranges of about 0.4 to 0.6 and 0.7 to 0.8 wt pct of SD2ES and SDS surfactant, respectively. However, for SDBS surfactant, the concentrations of sulfate used to compute percent wetting improvements are not accurate since this surfactant contains 20 wt pct Na2SO4 impurity. In reality, the SDBS contribution of Na2SO4 impurity provides considerable Na2SO4 in solution (depending on surfactant concentration) before any Na2SO4 is added separately. For example, there are 5.6 and 11.3 mmol/L of Na2SO4 in 0.4- and 0.8-wt-pct SDBS surfactant solutions, respectively, before any addition of electrolyte. Thus, the calculation of the percent wetting improvement after adding 15 mmol/L of Na2SO4 to an 0.8- wt-pct SDBS surfactant solution is really the difference between solutions containing 11.3 and 26.3 mmol/L of Na2SO4. This is likely an important wetting response factor, since the greatest response to Na2SO4 addition by anionic surfactant tends to occur at the lowest range of Na2SO4 concentrations. For example, in figure 6, for 0.14 wt pct SD2ES, a much greater wetting improvement occurs between zero concentration of Na2SO4 and 15.0 mmol/L Na2SO4 than between 11.0 and 26.0 mmol/L Na2SO4. This factor also explains the feeble wetting response of high concentrations of SDBS in figure 10 (curve B). High SDBS concentration results in excessive Na2SO4 concentration and, therefore, reduced wetting response.
Similar circumstances are also visible in figure 9 for SDS (curve A) and in figure 11 for SDHS (curve B). Therefore, it is safe to say that the slope of curve C in figure 17 would likely have been greater and aligned better with the SDS and SD2ES curves if a purer sample of SDBS had been available for testing.
Easy-to-Wet Coal
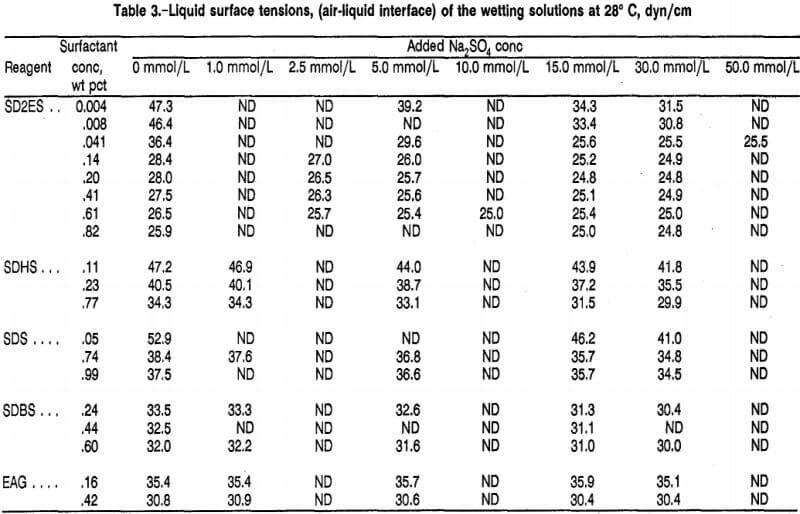
Figure 18 displays the wetting promotion of Na2SO4 as a function of the concentration of SD2ES, SDS, and SDBS anionic surfactants on easy-to-wet coal. As in figure 17, the wetting dependencies are expressed in percent wetting improvement of surfactant solution with added Na2SO4 (15.0 mmol/L) compared with the same surfactant solution without added Na2SO4. Figure 18 reveals that the promotion of wetting by 15 mmol/L of Na2SO4 was greatly different depending on the anionic surfactant used and its concentration. This result was dissimilar to results when anionic surfactant was applied to hard-to-wet coal, which showed a wetting promotion dependent chiefly on surfactant concentration and not on the anionic surfactant used (figure 17, curves B, C, and D).
SD2ES surfactant showed a maximum percent wetting improvement at about 0.61 wt pct surfactant concentration, while wetting improvement dropped off abruptly at lower and higher concentrations (fig. 18). On the other hand, SDS surfactant showed a gradual wetting improvement as its concentration was increased (fig. 18). For SDBS, the percent wetting improvement was greatest at low concentrations but dropped off rapidly until, at about 0.44 wt pct surfactant, wetting improvement leveled off, and higher concentrations had little effect on it.
In the case of SD2ES, higher surfactant concentration had little effect on wetting beyond the maximum. The wetting maximum for SD2ES suggests that 15 mmol/L of Na2SO4 may not be sufficient to compete with a high concentration of SD2ES surfactant ions for hydrophilic coal sites despite the valence advantage of sulfate ion in the ion-exchange reaction.
It is evident, then, that application of Na2SO4 to aid the performance of anionic surfactants applied to easy-to-wet coal is a complicated matter. Wettability testing is required to determine the optimum concentrations of surfactant and additive for a given easy-to-wet coal. In contrast, for hard-to-wet coal, application of Na2SO4 is always effective at lower concentrations of anionic surfactant.
Surfactant Coal-Wetting Performance and Surface Tension
Comparison of the ranking of wetting ability for the various surfactants with their ranked ability to reduce the surface tension of water indicates poor correlation except for SD2ES surfactant, which performed better than the other reagents in both reducing surface tension and wetting time (compare figure 19 with figure 14).
Hence, coal-wetting improvement through surfactant addition is not merely a function of a surfactant’s ability to reduce the surface tension of water. The surface tension does need to be reduced below 45 dyn/cm, but most surfactants will reduce the surface tension below this critical value easily at low concentrations. For example, less than 0.05 wt pct was required for SD2ES. If spontaneous wetting is achieved at 45 dyn/cm, why should further reduction in surface tension aid wetting beyond this point? Figures 6-7 and 9 affirm that higher concentrations of surfactant and Na2SO4 tend to improve coal wetting up to a concentration limit, but the limit occurs far beyond the concentrations giving critical surface tension.
Surface tension measurements of the wetting solutions do suggest the possible occurrence of close-packing phenomena at the coal-liquid interface. The reduced liquid surface tension from adding Na2SO4 to anionic surfactant solutions indicates that analogous close packing of surfactant molecules is occurring at the liquid-air interface. Table 3 shows the decrease in surface tension of anionic surfactant solutions as Na2SO4 is added, compared with the constancy of surface tension for EAG nonionic surfactant solution regardless of the presence of Na2SO4 at a given surfactant concentration. The surface tension reduction achieved for anionic surfactant through adding Na2SO4 is derived from improved close packing of surfactant at the air-liquid interface, caused by reduced electrostatic repulsion forces. On the other hand, the surface tension of nonionic surfactant solution and its wetting performance are unaffected by Na2SO4, since electrostatic forces are not operating in this case, and electrolyte addition will not affect close packing of molecules at either the air-liquid or coal-liquid interfaces. If anything, excessive Na2SO4 addition would tend to increase surface tension in this case, since concentrated electrolyte solutions (in the absence of surfactant) are known to increase surface tension above that for pure water.
It might be argued that close packing of surfactant molecules at the coal-liquid interface is wholly responsible for the improved wettability observed with Na2SO4 addition. However, close-packing phenomena on the coal surface would be predicted to affect wettability most noticeably at high anionic surfactant concentrations. But such was not the case in figure 6 (curves B, C, D, and E), where wetting improvements for high surfactant concentrations were modest or nonexistent upon increasing the Na2SO4 concentration.
A minimum surface tension of about 24.8 dyn/cm was achieved at about 0.20 wt pct SD2ES plus 30 mmol/L Na2SO4 (table 3). This value likely represents the point of ultimate close packing of surfactant at the air-liquid interface. Significantly, further SD2ES addition affected the wetting of the coals (positively or negatively) despite the likelihood of maximum close packing of adsorbed surfactant molecules at the coal-liquid interface. Therefore, these wetting changes must originate from the adsorption phenomena described earlier rather than from close-packing phenomena.
It is interesting to note that the minimum surface tension obtainable with SD2ES and Na2SO4 is approaching that of pure hydrocarbons of similar chain length, such as n-octane. One might surmise that the packing of surfactant molecules at the liquid-air interface is approximating molecular spacing. Thus, n-octane has a surface tension of 21.8 dyn/cm, while n-decane and n-dodecane are reported as 23.9 and 25.4 dyn/cm, respectively, at 20° C.
Conclusions
It was shown in this work that the favorable coal-wetting response previously achieved with SD2ES anionic surfactant, when adding a multivalent anion such as sulfate, was not an isolated case depending on a singularity of the SD2ES surfactant. Experiments with other anionic and nonionic surfactants indicated that improved wetting response for anionic surfactants in the presence of Na2SO4 was a consequence of their negatively charged character and, therefore, generically applicable to all anionic surfactants as a class. The limited response to Na2SO4 addition by nonionic surfactant further supported this conclusion.
Several factors were identified as having the potential to affect the wettability of coal by surfactants and by combinations of surfactant with electrolyte additive containing a multivalent anion. The factors are
- Reduction of the surface tension of water by surfactant or surfactant-additive combination;
- Adsorption of surfactant on hydrophobic sites of the coal surface to create hydrophilicity;
- Adsorption of surfactant on hydrophilic sites of the coal surface in reversed orientation to create undesirable hydrophobicity;
- Adsorption of multivalent anions, such as sulfate, on hydrophilic sites of the coal surface to preserve hydrophilicity;
- Close packing of surfactant molecules on hydrophobic sites of the coal surface to improve hydrophilicity; and
- Adsorption of surfactant to form hemimicelles on top of previously adsorbed surfactant to restore hydrophilicity.
The first factor was determined to be important only in reducing surface tension below a critical value for coal (45 dyn/cm). Below this value, liquid surface tension ceases to be important except that it may reflect the situation at the coal-liquid interface with respect to close packing of adsorbed surfactant molecules. However, in extensive testing with SD2ES surfactant, a maximum close packing appeared to be achieved on the coal surface (indicated by a relatively constant liquid surface tension), and yet wettability changed with further surfactant and Na2SO4 addition. Therefore, the close-packing factor 5 is considered of less importance than the adsorption factors 2, 3, and 4, which appear to dominate the wetting action of anionic surfactants on coal.
The hemimicelle formation factor 6 may also have some validity, but the applicability is tenuous since it is difficult to attribute certain experimental observations on hard-to-wet coal to this phenomenon while being unable to detect any presence of the phenomenon on easy-to-wet coal.
The anionic class of surfactants was more effective than nonionic reagents in wetting hard-to-wet coal. However, the performance of anionic surfactants can be inconsistent because of the electrostatic interactions between the charged coal surface and surfactant ions. Nevertheless, the employment of nonionic surfactants to avoid electrostatic interactions is not recommended. If nonionic surfactant adsorbs in adverse orientation or with poor close packing on the coal surface, nothing can be done to ameliorate the situation except to increase concentration (economically disadvantageous) or try a new surfactant. However, for anionic reagents, both of these conditions can be corrected by addition of an appropriate, inexpensive electrolyte such as Na2SO4. Nonionic reagents are regarded as ultimately more limited than anionics, since the electrostatic properties of the anionics can be manipulated advantageously with additives.
