Table of Contents
A molten salt, inert-atmosphere electrorefining process was developed for producing ductile vanadium from commercial vanadium. Five different chloride electrolytes were utilized. The chief impurity elements in the commercial vanadium were aluminum, iron, molybdenum, nitrogen, and silicon totaling 8 percent. Refining reduced these elements to produce 99.0 to 99.6 percent vanadium. The process was especially effective in eliminating nitrogen and oxygen to produce a ductile product. The chief contaminant of the refined vanadium was iron, ranging from 0.2 to 0.4 percent. Ingots prepared by arc melting the vanadium crystals were cold-rolled into 15-mil strips without annealing.
Laboratory production of ductile vanadium from commercial vanadium on a pound-a-day scale was accomplished in both the KCl-LiCl-VCl2 and CaCl2-NaCl-VCl2 electrolytes at 620° C. Ductile vanadium of 99.6-percent purity with a hardness of 35 Rockwell B was prepared using initial cathode current densities of 200 to 300 amp/ft². An 85-percent recovery of ductile vanadium was made in the KCl-LiCl~VCl2 electrolyte with an average current efficiency of 77 percent. The recovery of ductile vanadium in the CaCl2-NaCl-VCl2 electrolyte was 87 percent with an average current efficiency of 81 percent.
The feasibility of producing ductile vanadium by molten salt, helium atmosphere, electrorefining of commercial (90-percent-grade) vanadium was demonstrated.
The objective of this Bureau of Mines investigation was to develop a process for producing ductile vanadium by molten salt electrolyses from low-cost commercial (90-percent-grade) vanadium. This was one part of a Bureau research program for extracting and utilizing vanadium from vanadium concentrates such as vanadium carbides and vanadium oxides, and from metallothermically reduced metal containing 85 to 90 percent vanadium.
The potential uses of vanadium have not been fully developed because of the high cost, approximately $30 per pound, of the ductile metal. Ductile vanadium metal has been prepared by several methods. Harden and Rich produced ductile vanadium by reducing vanadium pentoxide with calcium and calcium chloride in a heated steel bomb. Van Arkel prepared ductile metal by the thermal decomposition of vanadium iodide. McKechnie and Seybolt used calcium reduction of vanadium pentoxide to produce a ductile vanadium.
Block, Brown, and Ferrante prepared the ductile metal by metallothermic reduction of vanadium chlorides. The use of molten salt electrorefining techniques to prepare high-purity vanadium has been reported by Cattoir and Baker and by Sullivan and Cattoir. Carbon, nitrogen, and oxygen are the major contributors to the nonductility of vanadium; Loria and coworkers and Ramsdell and coworkers have reported on the effects of these interstitial elements in electrorefined vanadium.
Vanadium of 90-percent purity has been available at quoted prices of $3.45 to $3.65 for 100-pound lots since 1954. This metal is produced by the aluminum reduction of vanadium pentoxide. The major impurities in the metal are up to 4 percent oxygen, 3 percent aluminum, 3 percent iron, 1.3 per-cent molybdenum, 0.8 percent silicon, and 0.1 percent nitrogen. In general, the 90-percent-grade material has a hardness of over 120 Rockwell B and contains 90 to 92 percent vanadium. It was apparent that a refining process would have to be developed to reduce the impurities and the hardness.
The criteria used for ductile vanadium were those of Rostoker, who stated that, to retain ductility, the oxygen plus nitrogen content must be kept below 0.20 percent and that carbon below 0.25 percent had little effect. Refining objectives were set at these values with an additional objective of hardness values below 90 Rockwell B.
Description of Equipment and Procedure
Electrolytic Cell
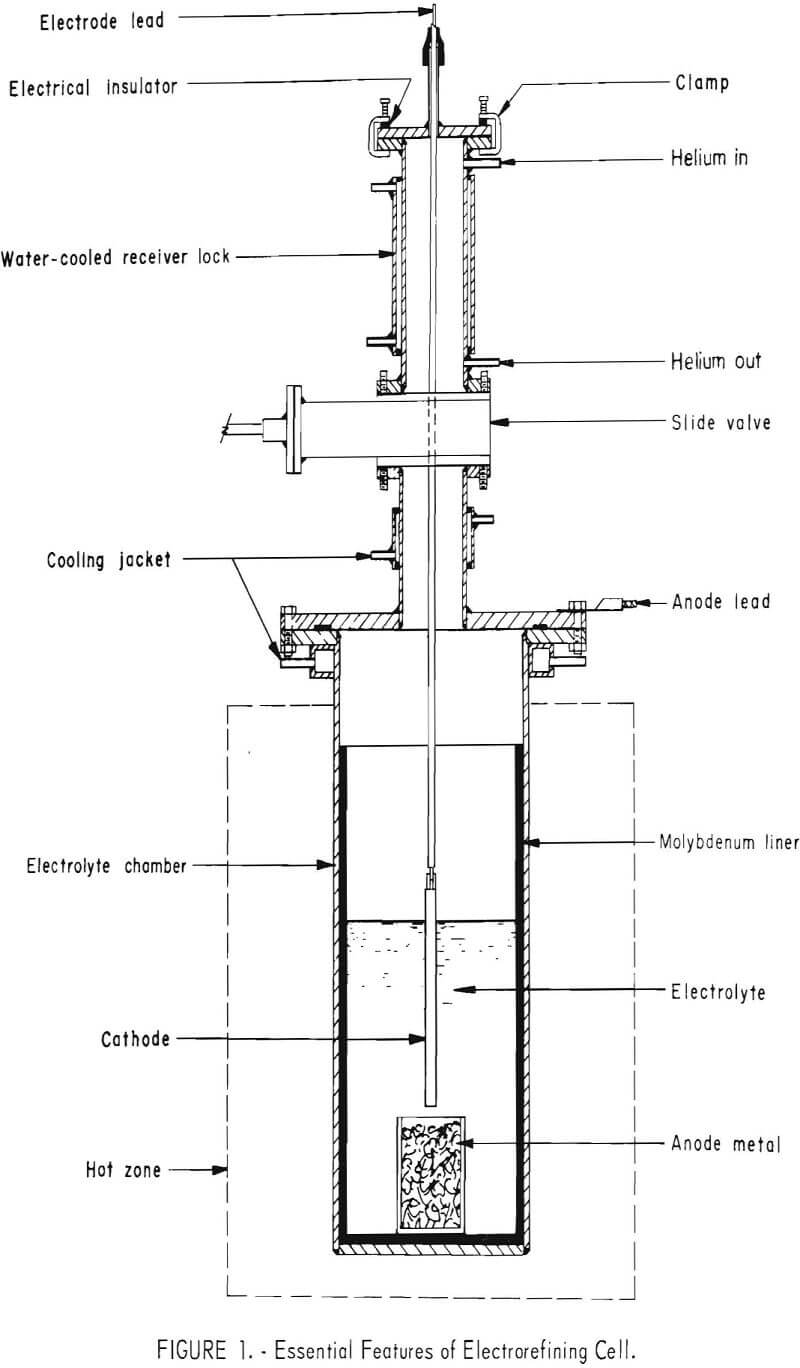
A drawing of a 12-inch-diameter, inert-atmosphere cell is presented in figure 1, and a bank of cells is shown in figure 2. The cell consisted of the following major components: A cell bottom that served as the electrolyte chamber was constructed of either Hastelloy C or N, ½ inch thick, 12-inch inside diameter, and 36 inches high. The top of the chamber was equipped with a water-cooled, mild-steel flange. A 12- by 24-inch-molybdenum or high-density-graphite liner was used as the electrolyte container in the chamber. A gastight, water-cooled slide valve provided the means of isolation between the electrolyte compartment and the receiver lock. The lock was equipped with connections for inert gas and for vacuum, and was water jacketed to cool the cathode deposits more rapidly. A metal cover with the cathode rod fitting and sight glasses for observation inside the cell were clamped on the top of the lock. Seals between various parts of the cell were made with flat or O-ring rubber gaskets, protected where necessary by cooling jackets.
A 25-kilowatt resistance furnace supplied heat to the cell and maintained the molten electrolytes at temperatures up to 1,000° C. The direct current used for electrolysis was supplied by a 12-volt, 100-ampere, full-wave selenium rectifier with variable output.
Operation
The assembled cells were leak-checked under both vacuum and pressure conditions. Electrolytes were prepared in the cell, using approximately 70 pounds of various alkali and alkaline earth chlorides combined with vanadium dichloride. Reagent-grade salts and chemicals were used. All the components of the particular electrolyte, except the vanadium dichloride, were charged into the cell, dried under vacuum, and melted under a helium atmosphere. All succeeding entries to and removals of materials from the cell were made under helium atmosphere control. It was necessary to maintain an inert atmosphere for two reasons: (1) the affinity of vanadium for oxygen and nitrogen; and (2) the oxidation of vanadium dichloride by air to form volatile oxychlorides.
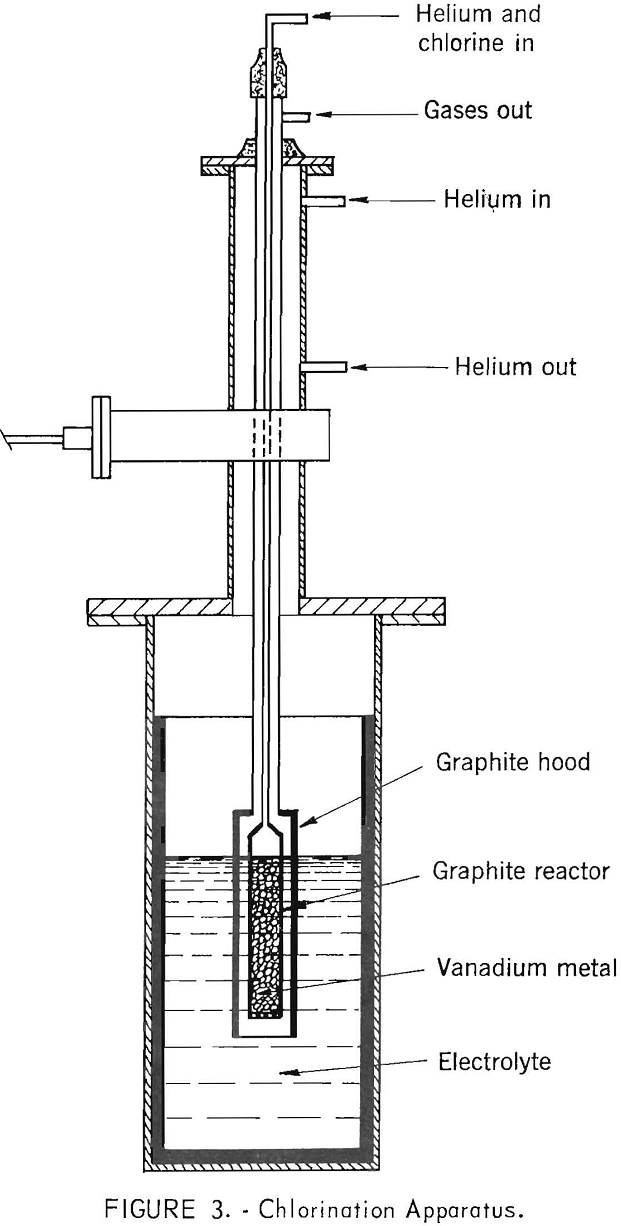
The vanadium dichloride used in preparing the electrolyte was produced in the cell. High-purity vanadium (99.9 percent) was contained in a graphite reactor and immersed into the molten salt. The metal was chlorinated to vanadium dichloride with a mixture of helium and chlorine. Generally, 5 to 6 pounds of chlorine were used to produce an electrolyte that contained from 12 to 18 percent VCl2. The time necessary for chlorination was approximately 3 hours. The chlorination was performed approximately 100° C above the melting point of the salt mixture. The vanadium dichloride dissolved in the molten salt to form the electrolyte. The graphite hood was used to protect the superstructure of the cell from hot, corrosive gases. Figure 3 is a drawing of the chlorination apparatus in place in the cell. The apparatus was withdrawn from the cell after chlorination was completed.
The vanadium used for refining in this investigation was supplied by the Vanadium Corporation of America. It consisted of minus 4- plus 8-mesh material which was placed on the bottom of the cell in retrievable graphite or molybdenum baskets. The anode was connected to the cell flange, and the cathode was connected to the cathode electrode lead rod. The cathodes were ¾-inch molybdenum rods, 10 inches in length. The mechanism of the electrolysis is represented by the following equations:
Anode area: V (crude) + 2Cl- → VCl2 + 2e-…………………………………………….(1)
Cathode area: VCl2 + 2e- → V (refined) + 2Cl-……………………………………….(2)
Net electrolysis: V (crude) → V (refined)……………………………………………….(3)
In this report one deposit is designated as a test. A series is a consecutive group of tests with a single anode addition. In a series, electrolysis was continual except for the time necessary to remove a cathode deposit. The cathode current density values reported were the initial cathode current densities.
When a test was completed, the cathode deposit was raised from the electrolyte until the electrical circuit was broken, and adhering salt was drained from the deposit for 10 minutes. The deposit was cooled to below 25° C in the lock before removal. The metal was stripped from the cathode, leached in dilute hydrochloric acid (1:20) to remove the entrained electrolyte, washed with water until free of chlorides, rinsed with acetone, and dried. The refined vanadium was screened to remove the minus 80-mesh fraction, which contained the bulk of the impurities. The minus 80-mesh fraction was not ductile but was suitable for refeeding to the cell as anode material.
The spent anode material removed from the cell was leached free of electrolyte, dried and sieved. The plus 8-mesh fraction, mostly unattacked metal, was suitable for refeeding. The anode sludge was discarded.
Preliminary Investigations
Various salt mixtures were investigated as electrolytes in developing an electrorefining process. The main factors considered in selecting a specific electrolyte were cost of component salts, temperature at which the electrolyte could be used, quality of refined metal, and ease of preparation and handling. Five electrolytes were investigated.
NaCl-VCl2 Electrolyte
Initial refining tests were made with a molten NaCl-VCl2 electrolyte in a cell equipped with a high-density-graphite liner. The electrolyte contained, in weight-percent, 88 NaCl and 12 VCl2. The electrolyte was operated at 820° C. The anode material was contained in a 6-inch-diameter graphite basket, 4 inches high, with ¾-inch walls. Its capacity was approximately 3 kg of anode feed. A cathode immersion of 2 inches was used.
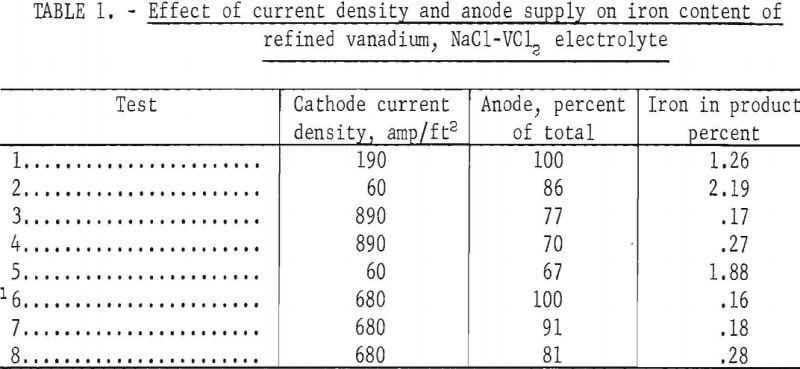
The first tests were made to determine the effect of cathode current densities, potentials, amount of anode, and ampere-hours of electrolysis on the quality of the refined vanadium. Cathode current densities between 65 and 900 amp/ft², potentials of 0.1 to 0.65 volt, and 15 to 595 amp hr of electrolysis were used. The best operating conditions were obtained using cathode current densities in the 500- to 700-amp/ft² range, potentials of 0.5 to 0.65 volt, and 260 amp hr of electrolysis. The tests indicated that iron contamination of the refined vanadium was related to current density and to the available quantity of anode material. The results are presented in table 1. Using 2.5 kg of anode material and low cathode current densities, vanadium with a high iron content was produced as shown in tests 1 and 2. By operating at high current density as in tests 3 and 4, the iron content of the vanadium, was reduced. The relationship between the available anode and the iron content of the refined vanadium is shown in tests 6, 7, and 8. Identical current densities were used in the successive tests, and the iron content of the refined vanadium increased as the anode material decreased.
Refining then was tested using the best conditions. In these tests, new anode material was added to the anode basket after each five tests. The results of the tests are summarized in tables 2 and 3, which give the operating conditions and the analysis of the feed, sludge, and products. The electrolyte dragout with the cathode deposits was excessive, as was the proportion of minus 80-mesh vanadium in the products.
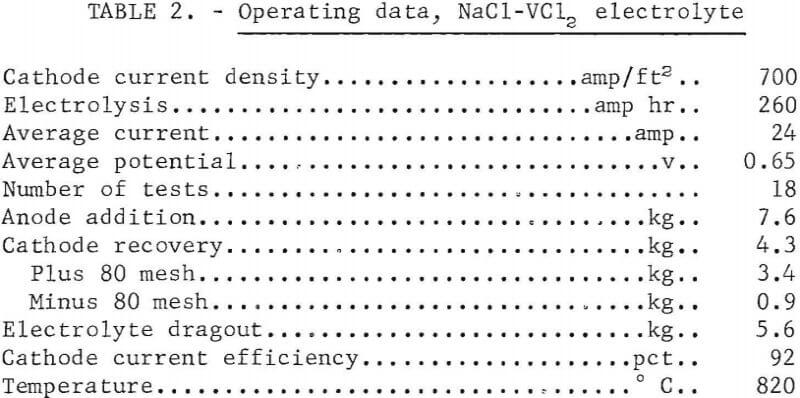
A ductile 99.5 percent vanadium was prepared in the plus 80-mesh fraction of the refined product. The metal had a Rockwell B hardness of 75 and represented 79 percent of the deposit. The minus 80-mesh fraction was not ductile but was suitable for refeeding to the cell. The amount of impurity accumulation in the anode is shown by the analysis of the anode sludge. The reason for an increase in the quantity of iron in the refined vanadium could well be the increasing iron content of the anode upon depletion of its vanadium content. The anode sludge was found by X-ray examination to consist of VO0.2 as the major constituent, with Al, VO0.9, VFe, and VC as minor constituents.
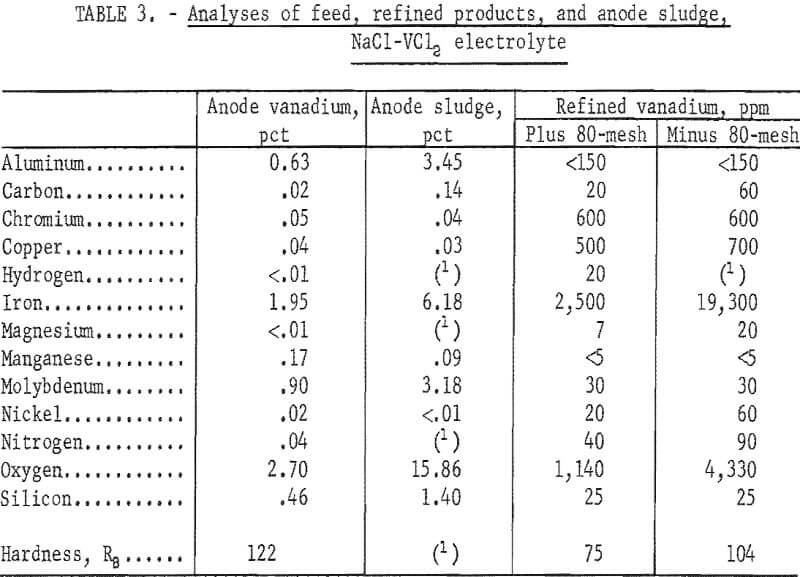
While 90 percent vanadium was refined in a NaCl-VCl2 electrolyte, an improvement was desirable in the product quality, particularly with regard to oxygen and iron contents.
LiCl-NaCl-VCl2 Electrolyte
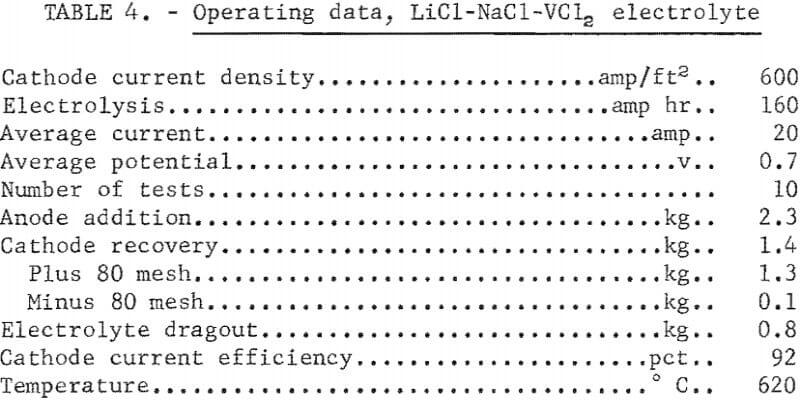
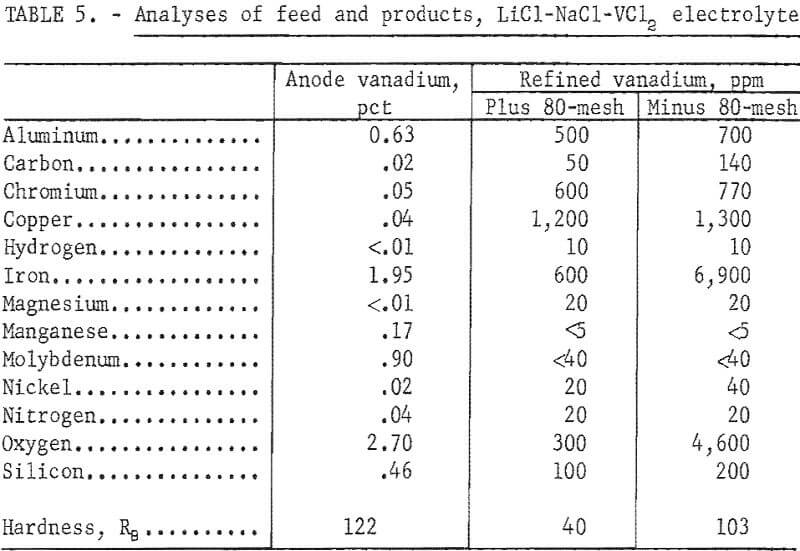
This electrolyte was prepared by adding lithium chloride (LiCl) to the NaCl-VCl2 electrolyte used in the previous operation, and contained, in weight-percent, 30 NaCl, 57 LiCl, and 13 VCl2. The electrolyte was operated at 620° C. Ten refining tests were made in this electrolyte with the same type of anode material used in the NaCl-VCl2 electrolyte and a cathode immersion of 2 inches. The operational data of the tests and the analyses of the products are presented in tables 4 and 5.
Iron and oxygen contamination were lower than in the products from the NaCl-VCl2 electrolyte, indicating that a lower electrolyte temperature contributed significantly to the decrease in the iron and oxygen content of the product. However, aluminum and copper increased in the refined vanadium. In addition, the minus 80-mesh fraction of the product decreased from 21 to 7 percent.
BaCl2-KCl-NaCl-VCl2 and KCl-LiCl-VCl2 Electrolytes
Parallel refining investigations were conducted in two 12-inch-diameter cells, one utilizing a BaCl2-KCl-NaCl-VCl electrolyte and the other a KCl-LiCl-VCl2 electrolyte. The first electrolyte contained, in weight-percent, 40 BaCl2, 25 KCl, 17 NaCl, and 18 VCl2. The operating temperature was 680° C. The second electrolyte contained, in weight-percent, 47 KCl, 37 LiCl, and 16 VCl2. The operating temperature was 620° C.
Molybdenum liners, 12 inches in diameter and 24 inches high, were used as the electrolyte containers instead of high-density-graphite liners. The anode containers were made of molybdenum sheet and had a capacity of 6 kg of vanadium feed material. Figure 4 shows the configuration of the anode basket. A 5-inch-cathode immersion was used.


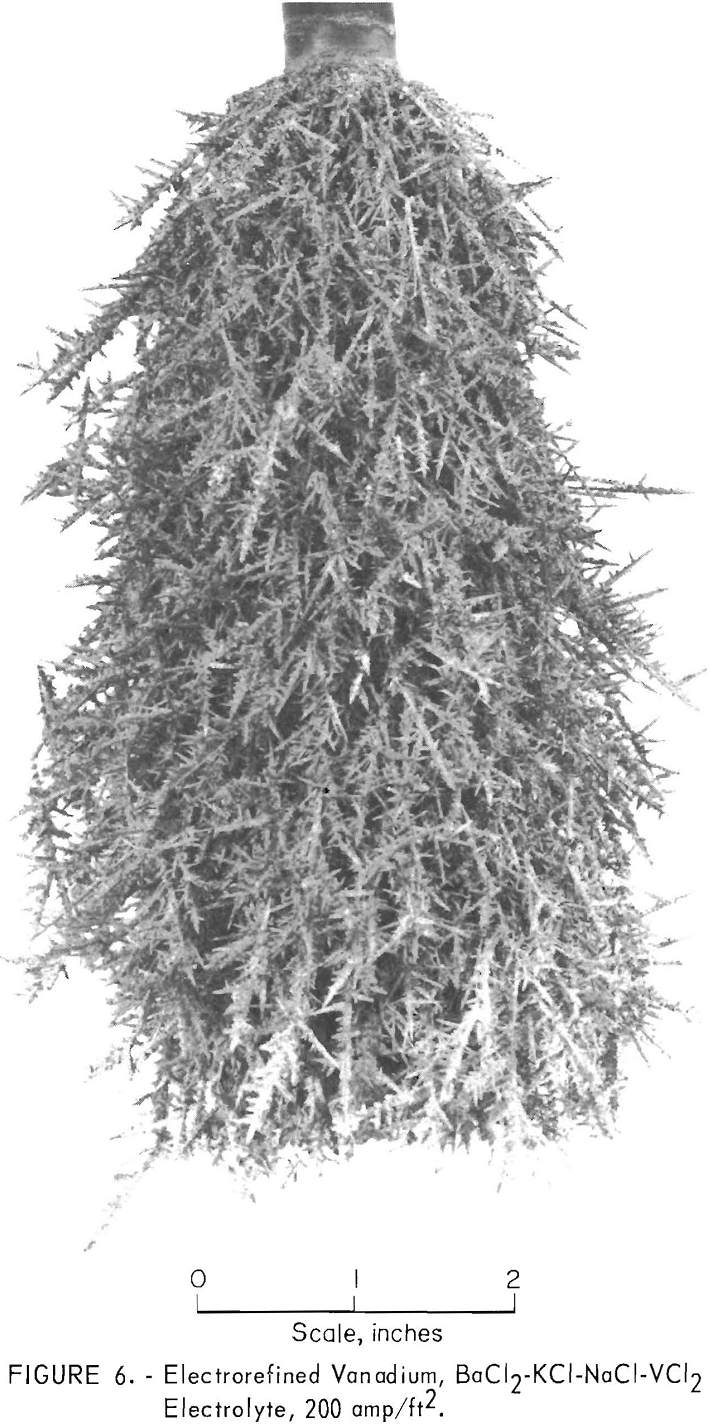
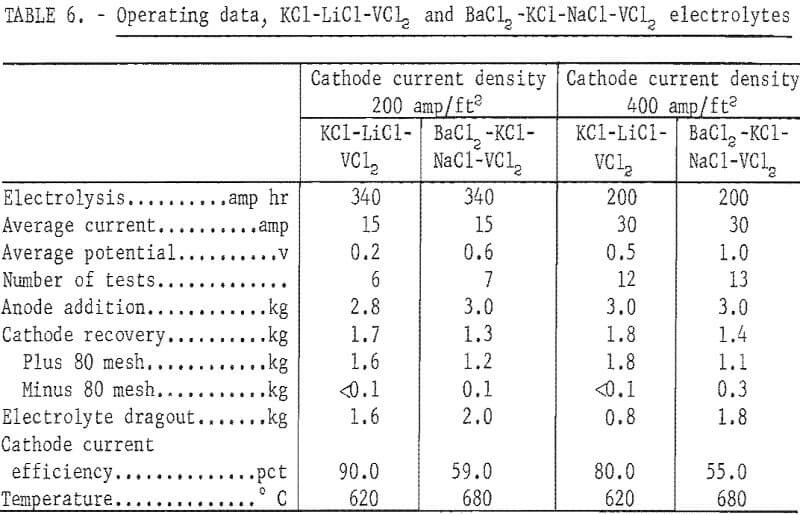
Two series of refining tests, one with a cathode current density of 200 amp/ft² and the other with 400 amp/ft², were conducted in each electrolyte. Table 6 compares the operational data. The KCl-LiCl-VCl2 electrolyte was superior to the BaCl2-KCL-NaCl- VCl2 electrolyte from the standpoint of dragout ratio, cathode current efficiency, and lower applied potential for a given cell current. Figures 5 and 6 show typical unleached deposits produced with a cathode current density of 200 amp/ft² in each electrolyte. These figures show that the vanadium crystals from the KCl-LiCl- VCl electrolyte were much coarser than those from the BaCl2-KCl-NaCl-VCl2 electrolyte.
Table 7 compares the quality of the vanadium anode and the plus 80-mesh refined products. Refining the 90 percent vanadium in either electrolyte generally yielded a product of better than 99.5-percent-pure vanadium. The elements that were substantially reduced were aluminum, iron, molybdenum, and oxygen. Although iron and oxygen were still the major impurities in the product, their proportions were much lower than in the product refined with a NaCl-VCl2 electrolyte. With the BaCl2-KCl-NaCl-VCl2 electrolyte, refining at the lower cathode current density resulted in a better product, especially with regard to iron content. The reverse was true for the KCl-LiCl-VCl2 electrolyte. Although the experimental range of cathode current densities was limited, these results indicated that the choice of cathode current density with respect to the electrolyte will be a factor in producing better metal.
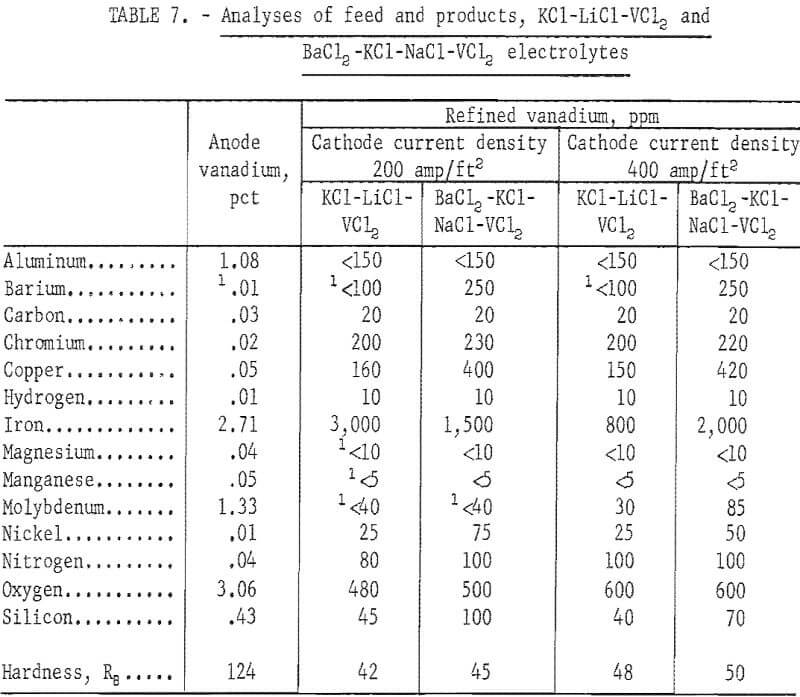
Copper was lowered in products from the KCl-LiCl-VCl2 electrolyte, but was about the same as the feed in the products from the BaCl2-KCl-NaCl-VCl2 electrolyte. Chromium was not eliminated to any degree with either electrolyte. The refined vanadium from both electrolytes contained up to 200 ppm of alkali impurities, and the products from the BaCl2-KCl-NaCl-VCl2 electrolyte contained 250 ppm of barium. Consolidation of the vanadium crystals into ingots by arc melting in an inert atmosphere reduced the barium content to 50 ppm and the alkalis to less than 5 ppm.
The minus 80-mesh product was 97 percent vanadium and contained iron, oxygen, and chromium as the chief impurities. The sludges recovered from each electrolyte averaged, in percent, 74 vanadium, 14 oxygen, 4 iron, 3 molybdenum, 3 aluminum, and 1 silicon.
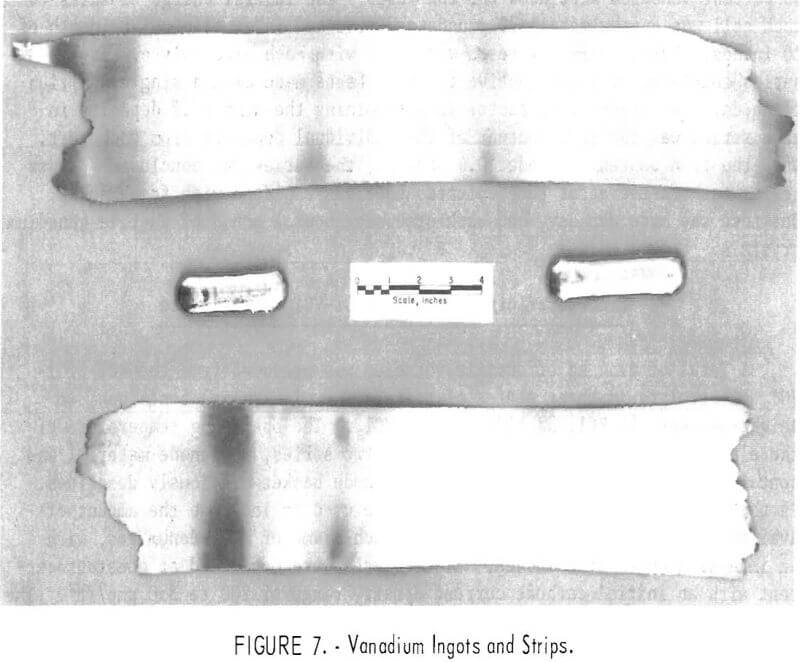
The low hardness values of the refined vanadium were an indication of its ductility. A composite of the metal from each electrolyte was consolidated into 120-g-ingots by inert-atmosphere arc melting. These ingots were cold-rolled into 15-mil-thick strips without intermediate annealing. Ingots and cold-rolled strips are shown in figure 7.
Laboratory-Scale Production Tests
The possibility of producing ductile vanadium by molten salt electrorefining in KCl-LiCl-VCl2 and CaCl2-NaCl-VCl2 electrolytes was demonstrated by two laboratory-scale production tests. One hundred pounds of commercial 90 percent vanadium were used for the tests. The initial charge of salts to each cell was approximately 70 pounds and resulted in an electrolyte depth of 10 inches. Three series of tests were made with each electrolyte. Each series consisted of a consecutive group of tests made with a single addition of anode. The controlling factor in determining the number of deposits in each series was the iron content of the individual deposits from each test. When the iron content exceeded 0.4 percent, the series was concluded. A new series was started after the depleted anode was replaced with fresh anode. One test was made per day, and each produced about 1 pound of ductile vanadium crystals.
Electrorefining in a KCl-LiCl-VCl2 Electrolyte
A Total of 11.4 kg of ductile vanadium was produced from the KCl-LiCl-VCl2 electrolyte in three series of tests. The electrolyte contained, in weight-percent, 47 KCl, 37 LiCl, and 16 VCl2. The operating temperature of the electrolyte was 620° C. In the first two series, the anode material was contained in the same type of molybdenum anode basket previously described. In the third series, two anode baskets were used to increase the amount of available anode. The cathode was a ¾-inch-diameter molybdenum rod, with an immersion depth of 8 inches. Electrolyses were conducted at constant current with an initial cathode current density range of 200 to 350 amp/ft². The voltage range was 0.4 to 0.7 volt. The average current and potential values are reported for each series of tests. Table 8 gives the operating data for refining in the KCl-LiCl-VCl2 electrolyte.
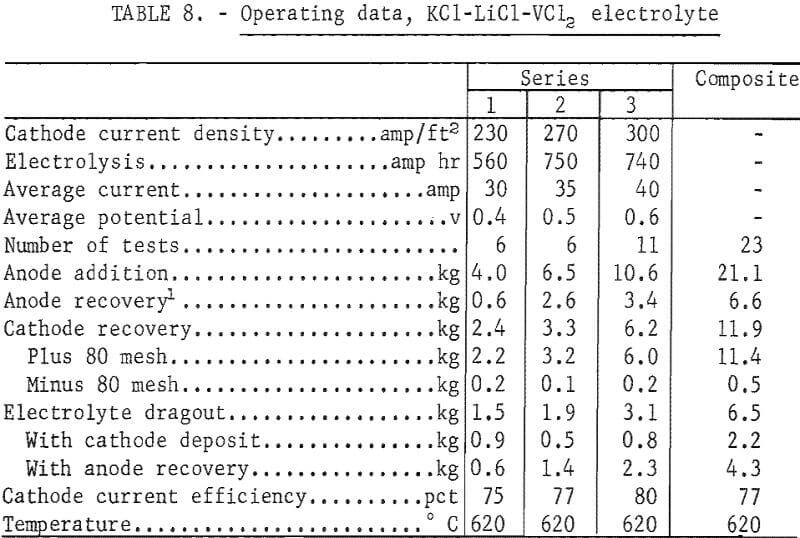
Eighty-five percent of the vanadium contained in the anode feed was recovered as ductile metal. Cathode current efficiencies ranged from 75 to 80 percent. The amount of electrolyte dragout at the cathode, in relation to the quantity of metal produced, decreased with an increasing amount of anode. About 60 percent of the vanadium present in each anode charge was refined before it became necessary to remove the anode basket, recover the unattacked anode material, and refeed it with new anode material. Figure 8 shows a typical deposit of 560 g of vanadium from the third series. The plus 80-mesh fraction of all three series of tests was composited to give a total ductile vanadium product of 11.4 kg. Table 9 shows the analyses and hardness values of the vanadium products.
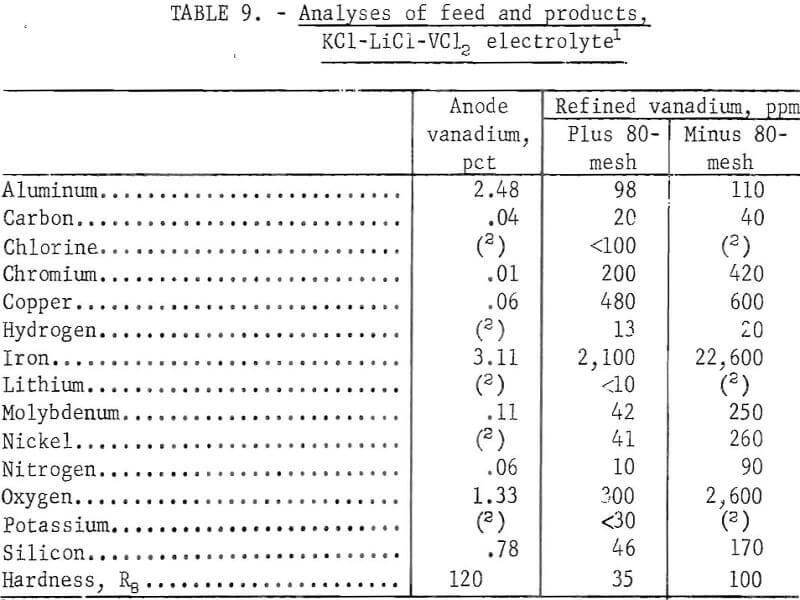
The major impurity elements present in the anode material were aluminum, iron, silicon, and oxygen. Electrorefining reduced all these elements, and the products had a total impurity content of approximately 0.35 percent. The oxygen and hardness values of the anode material and products show that the brittle feed material was converted to ductile vanadium. The minus 80-mesh product was suitable for refeeding as anode material. The anode sludge was similar in composition to that previously reported.

The initial electrolyte contained less than 0.01 percent of metallic impurities. During the three series of electrolyses the iron content ranged between 0.009 and 0.03 percent, and at the end of the tests the iron content was 0.009 percent. The chromium content of the electrolyte remained fairly constant at a concentration of 0.002 to 0.004 percent. The aluminum content of the electrolyte ranged up to 2.38 percent. Most of the aluminum that dissolved in the electrolyte was eventually sublimed out of the electrolyte and condensed as AlCl3 in the cooler portions of the cell. The impurity elements did not accumulate in the electrolyte to a degree that seriously affected the purity of the product.
Electrorefining in a CaCl3-NaCl-VCl2 Electrolyte
Three series of tests in the CaCl2 -NaCl-VCl2 electrolyte produced a total of 12.3 kg of ductile vanadium metal. The electrolyte contained, in weight-percent, 44 CaCl2, 39 NaCl, and 17 VCl2. The operating temperature of the electrolyte was 620° C. In the first series a molybdenum anode basket, similar to that shown in figure 4, held the feed material. In the second series two such baskets were used, and in the third series a concentric anode basket was used for the feed material container. Figure 9 shows the concentric anode basket. The cathode was a ¾-inch-diameter molybdenum rod, and cathode immersion depths were 5 to 8 inches. Electrolyses were conducted at constant current with an initial cathode current density range of 200 to 350 amp/ft². The voltage ranged from 0.5 to 0.7 volt. The average current and potential values are reported for each series of tests.
Table 10 gives the operating data for refining in the CaCl2-NaCl-VCl2 electrolyte.
Eighty-seven percent of the vanadium contained in the anode was recovered as ductile vanadium. Cathode current efficiencies ranged from 72 to 86 percent. A typical deposit of 465 g of vanadium from the second series is shown in figure 10. A comparison of the first and third series shows that the recovery of metal from the same amount of anode was higher in the third series. Table 11 compares the test products obtained in these two series with different anode configurations. The iron content and the hardness of the plus 80-mesh products were used as the comparison basis.
The concentric anode basket was superior to the single circular basket in metal recovery, proportion of plus 80-mesh product, lower cell voltage, electrolyte dragout with the deposit, current efficiency, lower iron content of refined vanadium, and better ductility. These results were attributed to a larger effective anode area and better access of the electrolyte to the anode material with the concentric basket. The concentric basket was designed to hold 12 kg of vanadium anode, but to make series 1 and series 3 comparable only 6.7 kg were used. With a full charge of anode the differences between the two series would have been even greater. Figure 11 shows a typical deposit of 355 g of electrorefined vanadium produced with the concentric basket. The crystal dendrites were larger than those produced in the other two series.
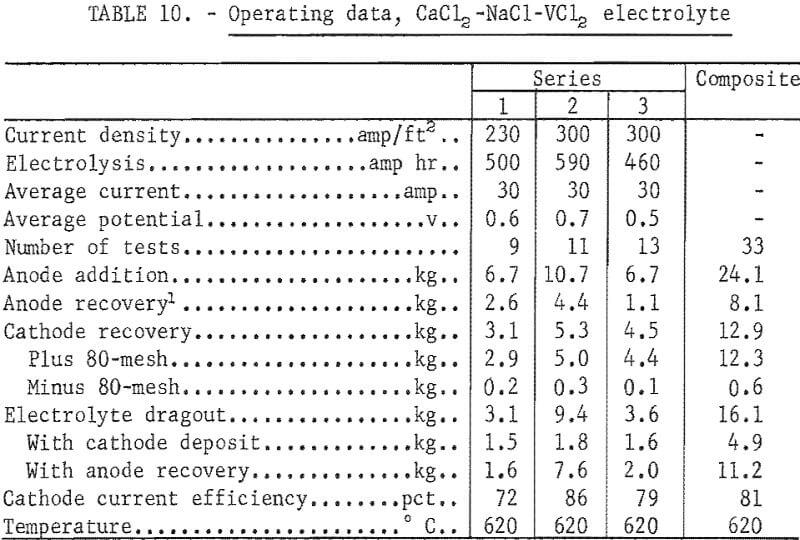
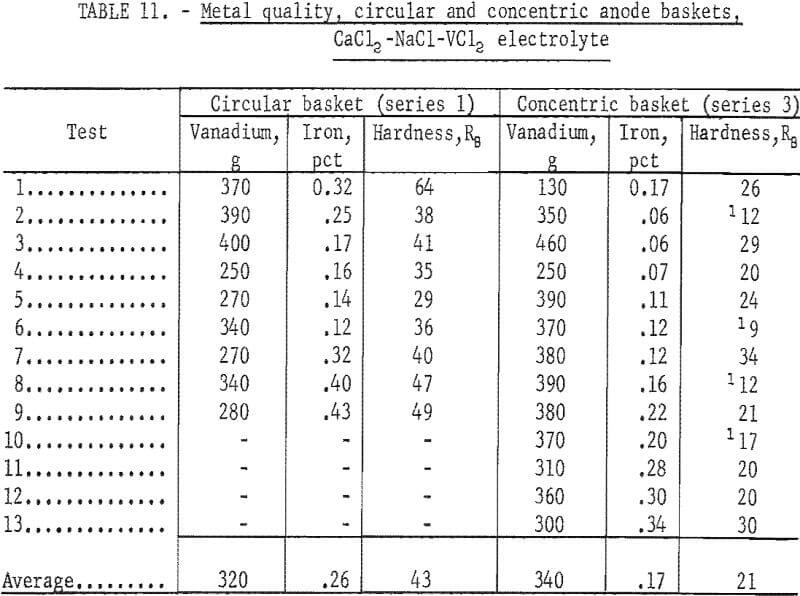


The results of varying the amount of anode in similar containers are seen by comparing the first and second series. The average iron and hardness values of the second series were 0.21 percent and 38 Rockwell B, respectively.
Series 2, with a larger amount of anode material, was better than series 1 in metal recovery, cathode current efficiency, electrolyte dragout with the deposit, proportion of plus 80-mesh product, lower iron content of refined vanadium, and lower hardness values.
Table 12 shows analyses and hardness values of the feed material and the composited refined product of the CaCl2-NaCl-VCl2. The product contained approximately 0.35 percent of impurities and was similar to that from the KCl-LiCl-VCl2 electrolyte. It contained slightly less iron and slightly more of other impurity elements. Both products had hardnesses of 35 Re, indicating equal ductility. Anode sludges were similar to those previously obtained.
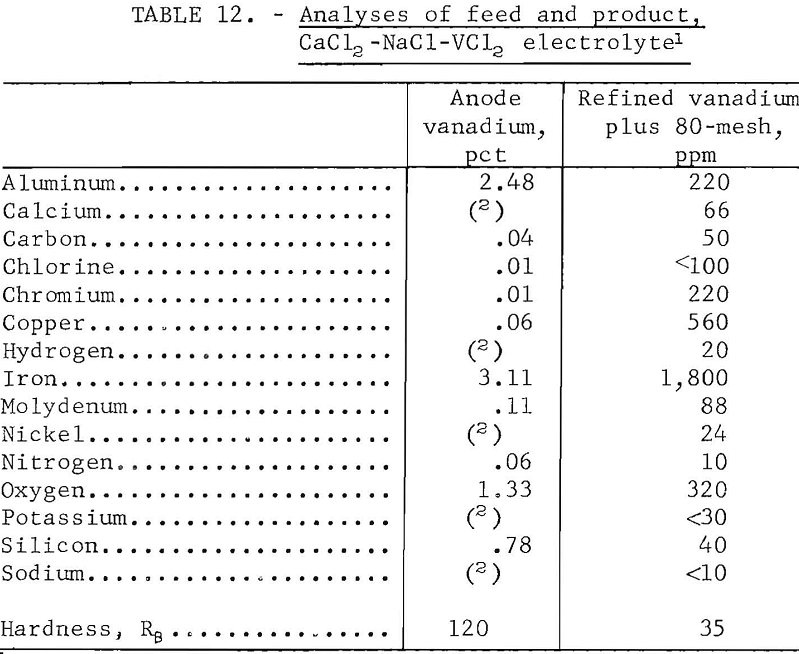
The CaCl2-NaCl-VCl2 electrolyte was quite similar to the previous electrolyte with respect to the accumulation of the impurities. The three major impurity elements that transferred to the product were iron, chromium, and copper. These were found in the electrolyte in the same range as in the KCl-LiCl-VCl2 electrolyte. Aluminum also dissolved in the electrolyte but sublimed out on the cooler parts of the cell.
In both of the electrolytes, the recovery of 99.6 percent ductile vanadium was limited primarily by the iron content of the commercial feed material. The extraction of vanadium would be increased by the use of feed containing less iron. Producers of commercial vanadium have indicated that the iron content of their material can be held below 1 percent. The electrorefining of vanadium prepared by the aluminum reduction of vanadium pentoxide and containing less than 1 percent iron has been reported.
