If a charge of binary solid-solution alloy is melted and then frozen slowly from one end, as for example in the Bridgman method of making single crystals, coring usually occurs, with a resulting end-to-end variation in concentration. Such coring, or normal segregation, is undesirable where uniformity is an object. On the other hand, for certain systems, it can be utilized to refine a material by concentrating impurities at one end of the ingot.
Normal Freezing
Before considering zone-melting, segregation during normal freezing will be reviewed briefly. If a cylinder of molten binary alloy is made to freeze from one end, there usually will be a segregating action which will concentrate the solute in one or the other end of the ingot.
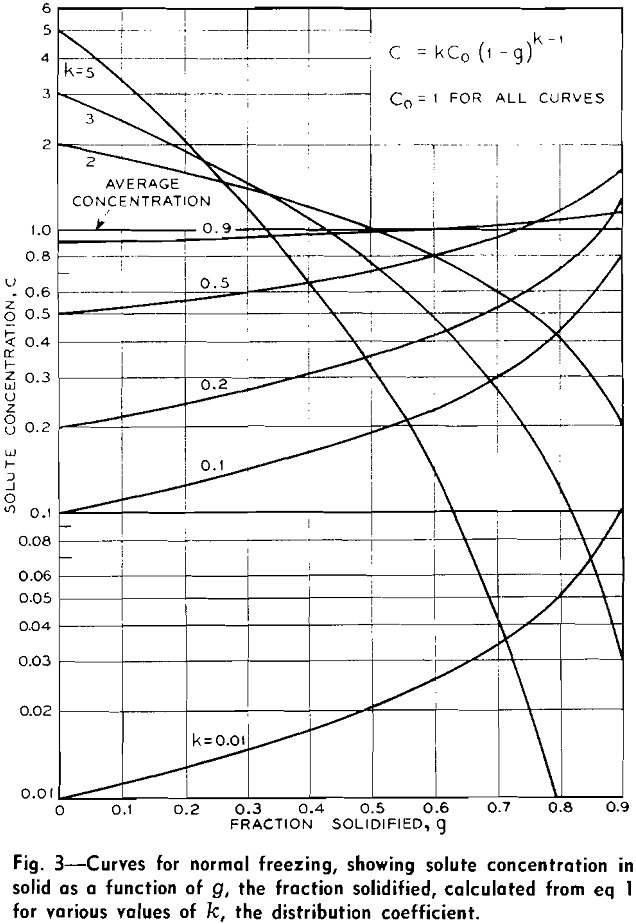
The concentration in the solid as a function of g, the fraction which has solidified, can be expressed by the relation:
C = kCo (l-g)k-1……………………………………………….[1]
where Co is the initial solute concentration in the melt. Eq 1 is based on the following assumptions:
1—Diffusion in the solid is negligible. 2—Diffusion in the liquid is complete (i.e., concentration in the liquid is uniform). 3—k is constant.
Concentration curves representing eq 1 for k’s from 0.01 to 5.0 are plotted in Fig. 3. This equation, in one form or another, has been treated by Gulliver, Scheuer, Hayes and Chipman for alloys and by McFee for NaCl crystals.
Zone-Leveling Processes
The processes of this part are designed to produce a uniform, or level, distribution of solute in the ingot.
Single Pass: Consider a rod or charge of alloy whose cross-section is constant and whose composition, Co, is constant, although permissibly varying on a microscopic scale. Such a charge might be a rapidly frozen casting or a mixture of crushed or powdered constituents.

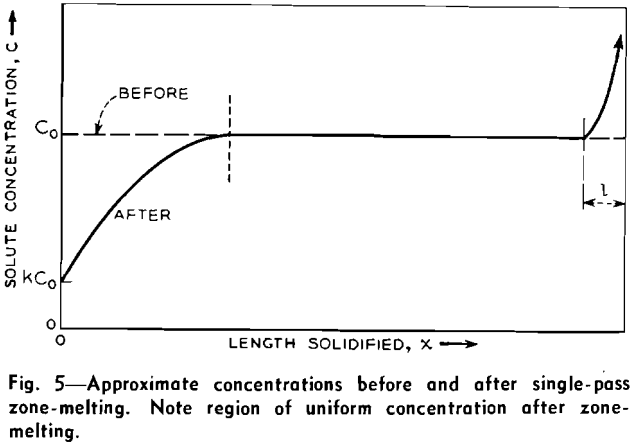
As the molten zone advances it freezes out a layer dx of solid and melts a layer dx of the charge. The first solid to freeze, at x=0, is of concentration kCo. For k<l, kCo is less than Co and hence the liquid is enriched. As the zone progresses the liquid continues to be enriched, although at a decreasing rate, until it attains concentration Co/k.
The concentration C at any length x in the zone-melted bar (except in the last zone) is given by:
C/Co = l – (l-k)e- kx/L………………………………………………………………[2]
where x is the length solidified, measured from the starting end, and not the fraction solidified.
Calculated curves of C vs. x/l according to eq 2 are plotted in Fig. 6 for k’s from 0.01 to 5.0, for the particular case of a charge ten units long, unit zone-length, and unity Co. Nine zone-lengths are depicted; the tenth solidifies by normal freezing.
Reverse Pass: It has been shown that zone-melting of a uniform rod results in two end-regions in which C differs considerably from Co. An improvement in uniformity can be made by re-zone-melting in the reverse direction, from right to left.
Repeated Pass: If a sufficient number of back-and-forth passes is made in a straight bar, all deviations from a uniform composition can be eliminated, except in the last zone to freeze. For a starting charge of mean composition Co, not assumed to be uniform, and a charge of total length d, the final concentration, Cf of the uniform rod is given by:
Cf/Co = dk/dk + L (l – k)………………………………………………………….[3]
The final concentration in the molten zone will be Cf/k
Starting Charge: The initial transition region in single-pass zone-melting is a result of the fact that the molten zone must travel some distance before it accumulates enough solute to attain concentration Co/k.
Zone-Refining Processes
The processes of this part have the object of purifying a solvent material, or separating solute from solvent.
Utilization of segregation during normal freezing to purify part of an ingot is well known. The process is particularly effective where k is small and diffusion in the solid is limited.
The particular merit of zone-refining becomes evident when repeated crystallizations are desired. By passing a bar through a series of heaters a number of molten zones can be made to traverse the charge in a single operation. Each zone picks up solute on its way through the charge and deposits it at the end of the charge. Thus, any desired number of refining steps can be obtained in a single operation, and without resort to the intermediate steps of cropping.
Discussion
Possibly the most significant feature of zone-melting is its flexibility. The charge may be regarded as a medium and the molten zone as a distributor of solutes in the medium. Subject to the restriction that the concentration freezing out of the molten zone is k times that in the zone, and to certain other restrictions on solidification conditions, an operator can produce a large variety of useful distributions of solute in an ingot. Among the tools at his disposal are the arrangement of the starting charge, and the size, number, and direction of travel of the molten zones.

