 Acid Rock Drainage as a concern and part of all metals and mining operations. ARD is often mislabelled AMD or Acid Mine Drainage too. So, to better understand AMD and ARD, let’s begin by defining some terms like pH -The standard measure of acidity.
Acid Rock Drainage as a concern and part of all metals and mining operations. ARD is often mislabelled AMD or Acid Mine Drainage too. So, to better understand AMD and ARD, let’s begin by defining some terms like pH -The standard measure of acidity.
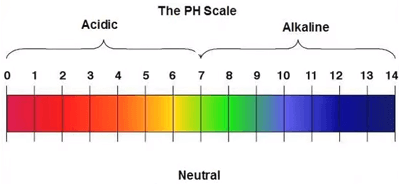 The pH scale actually measures hydrogen ion concentration in water solutions.
The pH scale actually measures hydrogen ion concentration in water solutions.
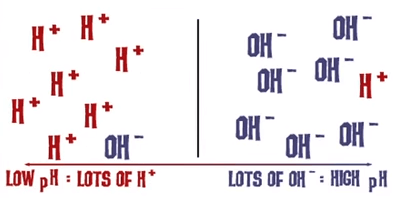
 Pure neutral water has a pH of 7, acids have a pH less than 7 and outgoing solutions a pH greater than 7.
Pure neutral water has a pH of 7, acids have a pH less than 7 and outgoing solutions a pH greater than 7.
 Like the decibel scale for noise the pH scale is logarithmic.
Like the decibel scale for noise the pH scale is logarithmic.

A small change in pH represents a large change in age plus concentration.
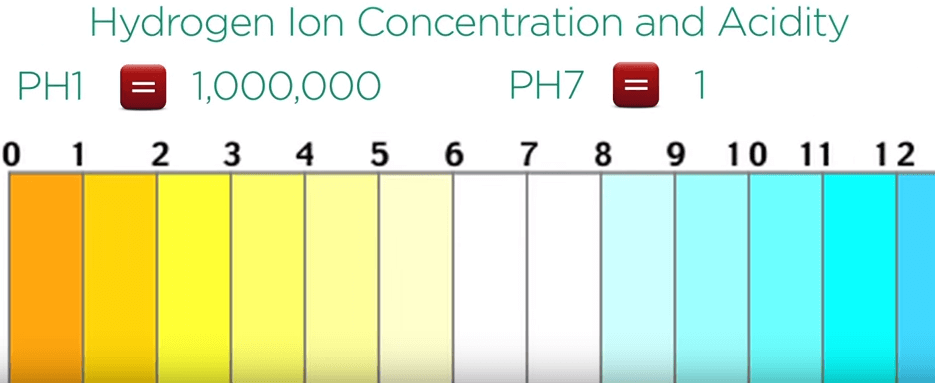
pH 1 has hydrogen ion concentration and acidity 1 million times higher than pH 7.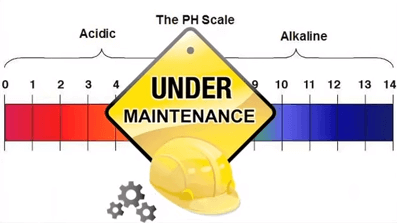
The maintenance of a desired pH range is important for living organisms, agriculture, industry and the environment.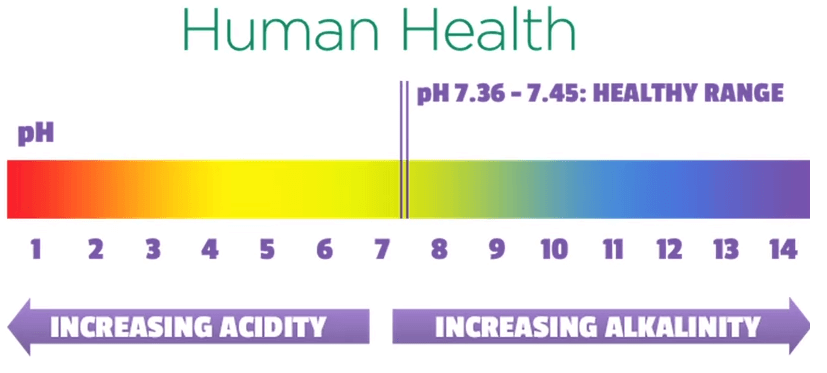
For example human health depends on the blood pH being within a narrow range.
Plant growth can be severely affected if pH of soils, rivers and lakes are too high or too low.
Metal items like steel pipes and pumping equipment can be corroded quickly if they carry acidic water.
Some solutions resist changes to pH and are called buffers, they tend to act like sponges for H+ and prevent water from becoming acid.
Fluids and living organisms and seawater have strong buffering potential to prevent acids from developing, in contrast pure rain water is poorly buffered.

 Substances like lime that increase the buffering capacity of water are called bases.
Substances like lime that increase the buffering capacity of water are called bases.
 Calcite is the mineral form of lime and limestone is a rock made of calcite.
Calcite is the mineral form of lime and limestone is a rock made of calcite.
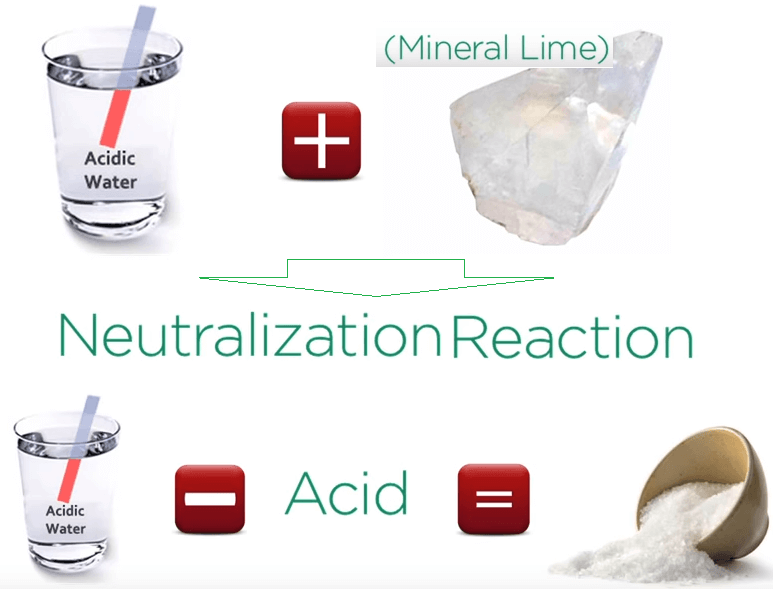 When acid waters and base like calcite are combined, a neutralization reaction in which the water is de-acidified and salt is produced.
When acid waters and base like calcite are combined, a neutralization reaction in which the water is de-acidified and salt is produced.
The natural pH of water and soil varies around the earth.
Acid soils are most often found in areas of high rainfall like jungles and forested areas while desert soils are alkaline.
This is because rain water naturally removes CO2 from the atmosphere forming a weak acid with a pH of 5.7. The naturally acidic rain then weathers rock and soil which also lowers soil pH.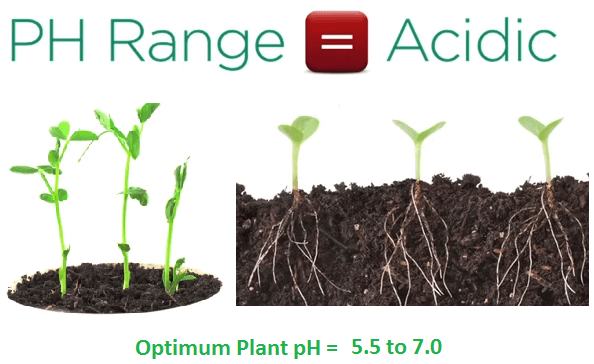
Natural plant root activity then further acidifies soils as minerals like iron required for growth are more soluble in acids as a result the optimum pH range for most plants is acidic between 5.5 and 7.0.

Acid rock drainage: Metals rarely occur in their pure state but are often formed in nature combined with sulfurous minerals called sulfides.
Metals are not usable as sulfides and after mining the sulfide minerals, sulfur needs to separate and be removed to produce a pure metal.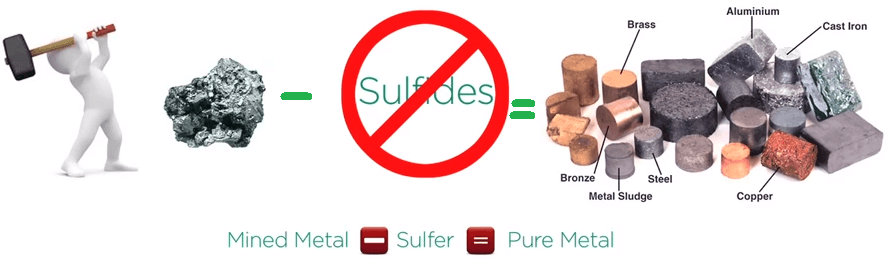
This process takes human energy but if we’re lucky nature has already done the work for us.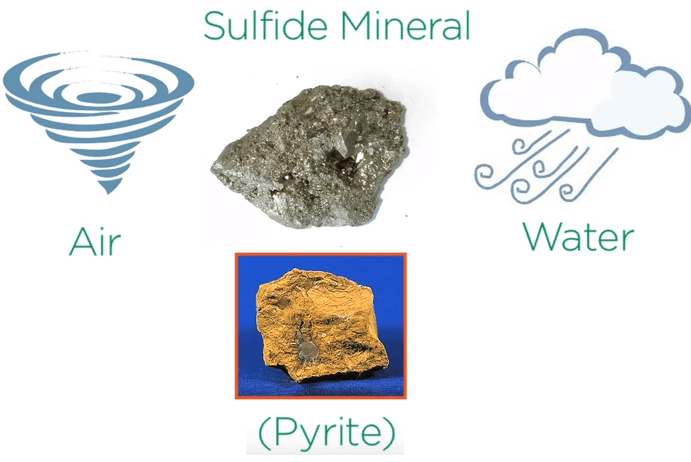
When sulfide minerals are exposed to air and water they corrode. Take for example the iron sulfide pyrite which is the most common sulfide mineral.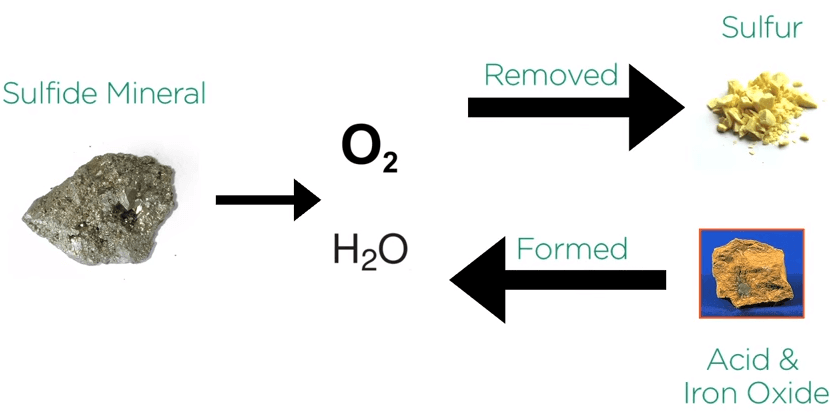
When exposed to atmospheric oxygen and water, sulfur is removed as acid in iron oxide a rust material is formed.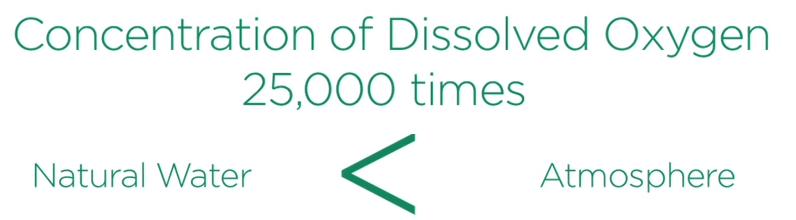
Sulfides located below the water table or under water will not weather significantly because the concentration of dissolved oxygen in natural waters is approximately 25,000 times lower than found in the atmosphere.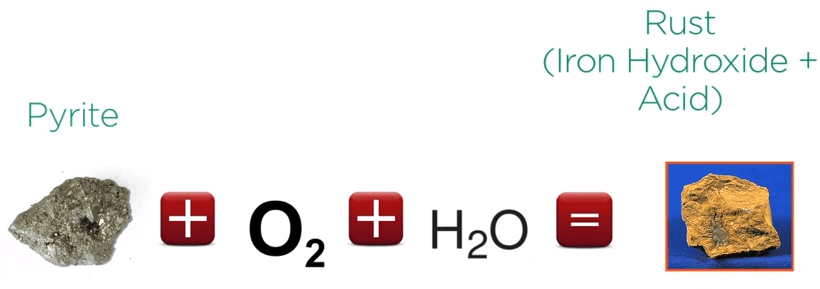
Pyrite plus oxygen plus water equals rust or iron hydroxide plus acid.
Where sulfides have been exposed to weathering for a long time in areas for example in Nevada or Chile natural can remove all the sulfur for us.
This kind of weathering is often helpful for gold and copper as these metals can be more cheaply removed from oxidized rock now devoid of sulfur.
The reverse is often true for silver, lead and zinc which are generally more inexpensive to refine into metal from sulfides then oxide minerals. The weathering of sulfides is a natural phenomenon that is happening all the time.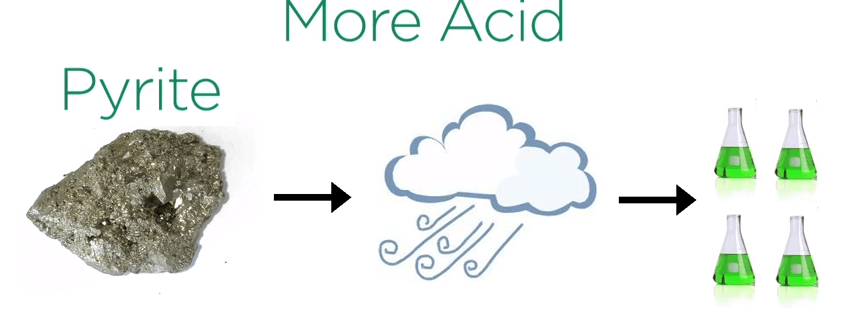
Pyrite is the most common sulfide mineral on earth and occurs over vast areas generally without more valuable sulfide minerals.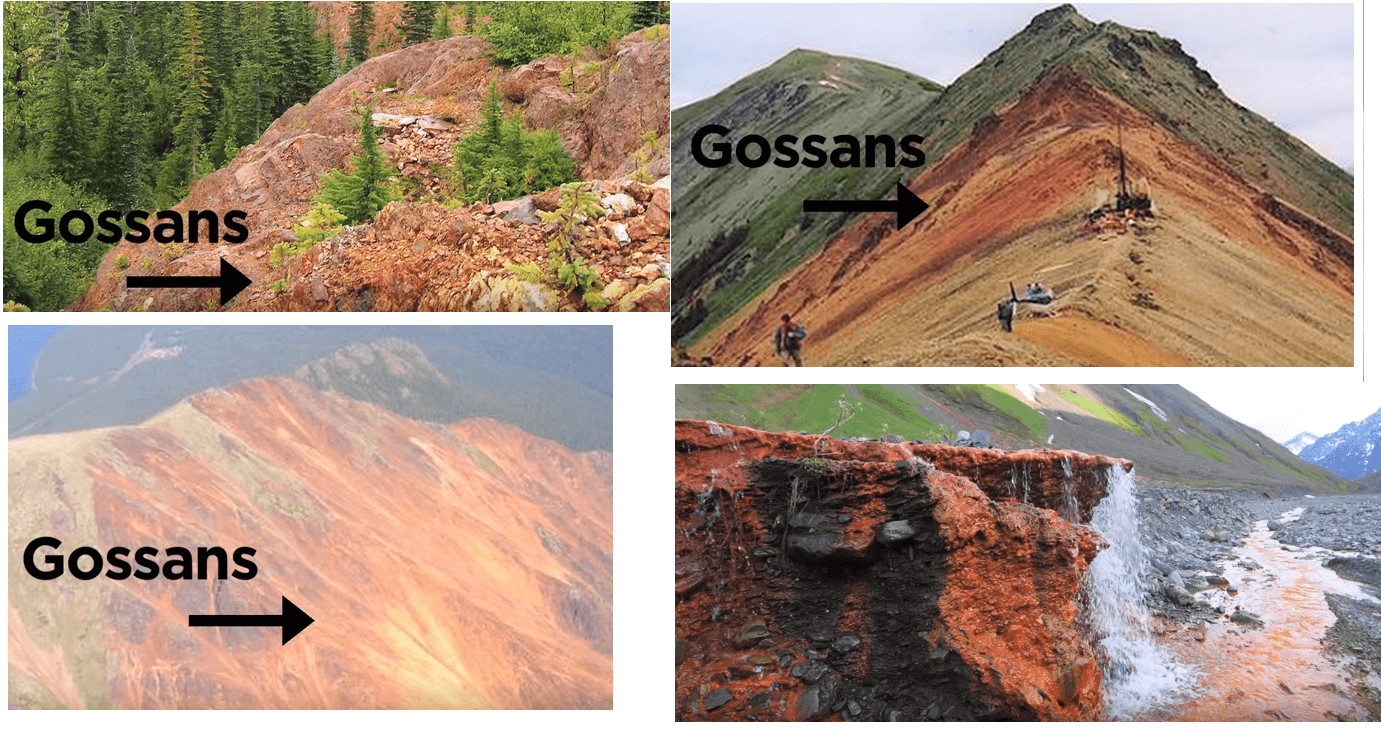
Pyrite produces more acid than other sulfide minerals when weathered and is generally the cause of rusty rock exposures called Gossans. They can be seen on mountain ridges, road cuttings and other excavations but rust isn’t the only signal that weathering sulfides give to the exploration geologists looking for metal.
The acid created by weathering sulfides can dissolve metals out of the rock like copper and zinc and can naturally turn streams and even rivers acidic.

To explore for sulfide deposits geologists look for naturally acidic creeks with high metal content.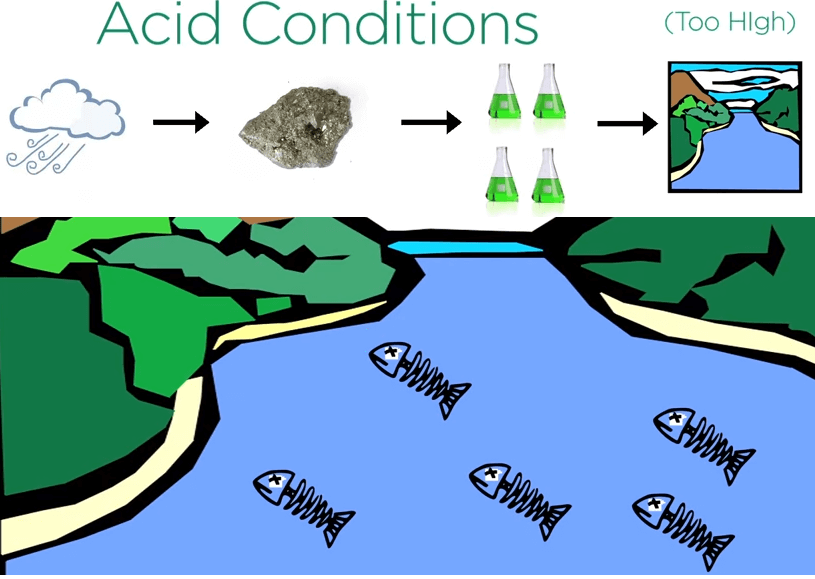
Weathering sulfides can create acid conditions and metal levels in streams so high that fish and other organisms can’t live in them.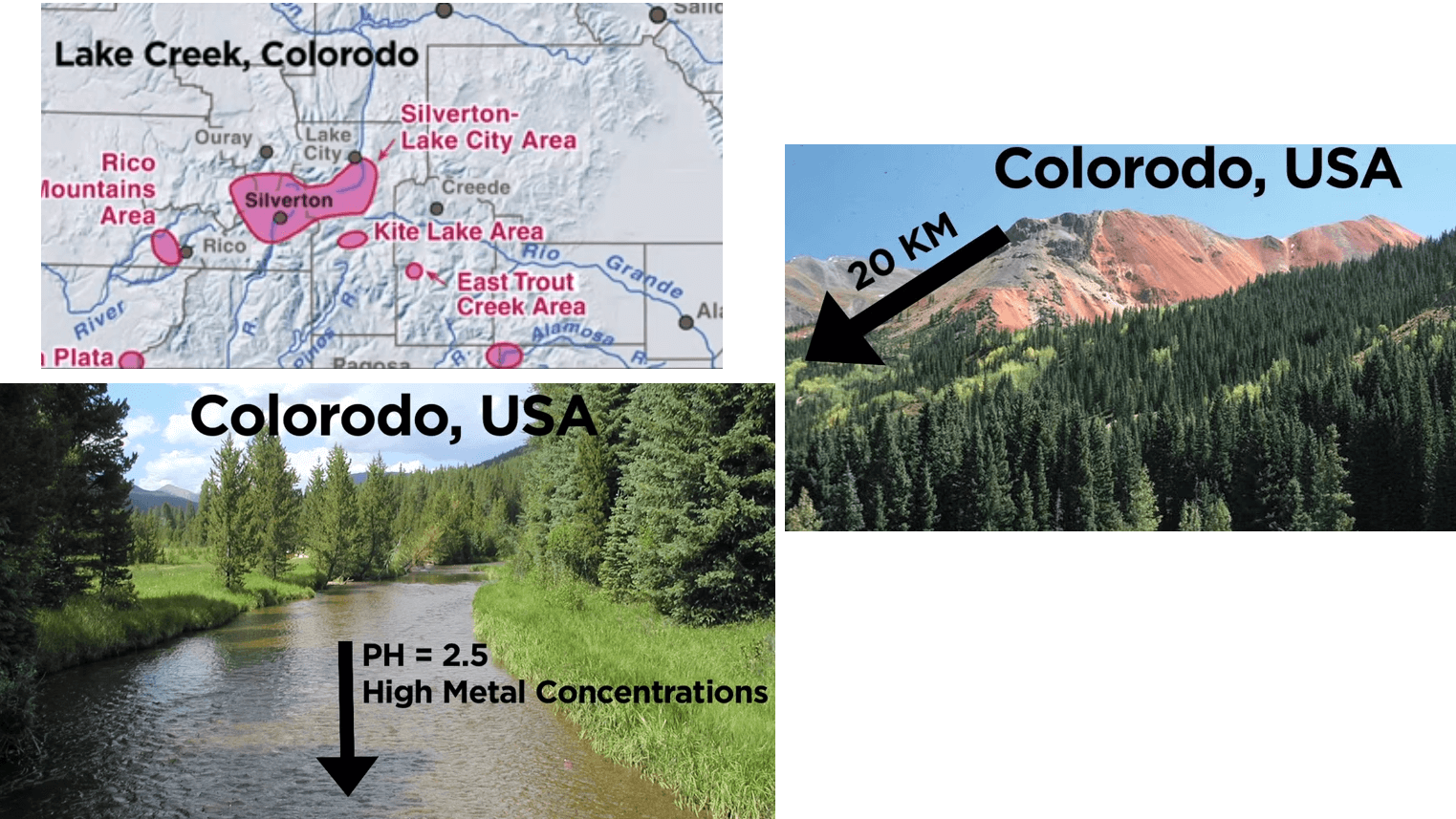
For example in Colorado Lake Creek, watershed natural acid rock drainage has developed from large exposures of pyrite on a mountain. There are over 20 km downstream from the red Gossans, pH values as low as 2.5 occur in the stream along with high dissolved metal concentrations.
Locally this has had severe adverse impacts to aquatic life.
Nevertheless the water and sediment quality of Twin Lakes Reservoir 27 km downstream is sufficiently diluted and buffered to support Trout Fishery and remnants of acid rock drainage are negligible.

Acid rock drainage and mining: Excavation like mining can accelerate the process of acid rock drainage because it local exposes more sulfide minerals to atmospheric oxygen.
Historically in construction, road building and mining industry there was limited recognition of this potential problem and there are a number of sites and old mines where increased acid rock drainage was created by excavation.
For example the Halifax Canada International Airport was built on sulfide rocks which when exposed locally increased natural acid rock drainage.
Many older mines in the world also added to natural acid drainage and resulted in impacts on the environment.

Although it is often difficult to know how much acid is naturally produced and has resulted from mine disturbance. For this reason acid rock drainage is often raised as a concern when new mines are proposed.
The presence of sulfides does not guarantee that acid rock drainage will happen as many rocks contain sufficient buffering capacity to limit or prevent acid development from the sulfides present.
Nevertheless in the last 20-30 years a much better understanding of the potential for acid drainage as evolved. Strategies for preventing acid development are implemented in all modern industrial mining operations.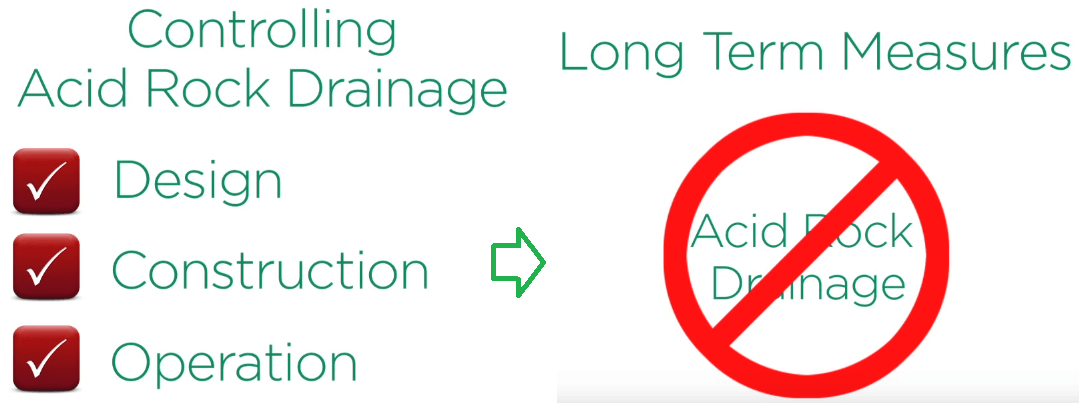
Where acid rock drainage is likely to occur its control is a primary objective in the design construction and operation of the rock entailing storage facilities with established long term measures to prevent acid development and treat water when it is necessary.
Treatments include such measures as adding buffer materials like limestone limiting the area of exposed acid generating rock and compacting acid generating rock to reduce water infiltration and covering intermediate ceiling layers.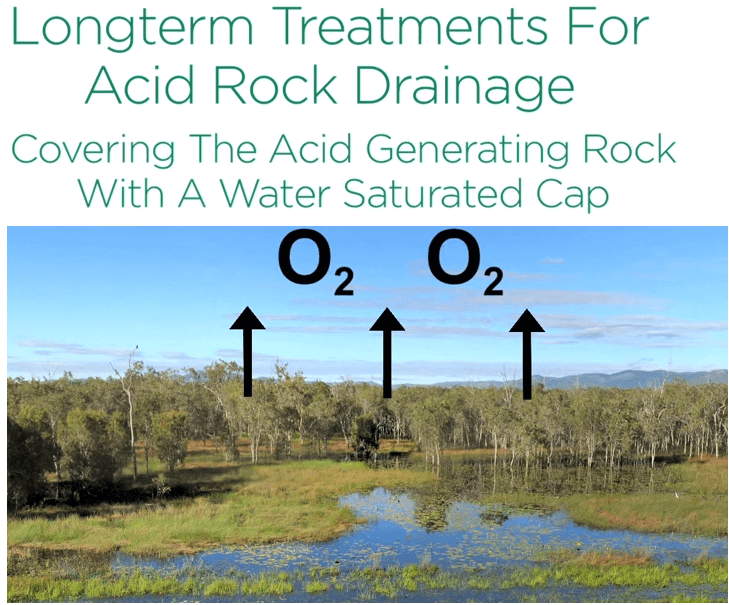
In the long term acid drainage control is achieved by covering the acid generating rock with a water saturated cap like a wet land keeps out oxygen.
 By closing monitoring and balancing mine water with natural rain and ground water, modern mines ensure that acid rock drainage is controlled long after mines are shut down.
By closing monitoring and balancing mine water with natural rain and ground water, modern mines ensure that acid rock drainage is controlled long after mines are shut down.
 Mining removes and sequester sulfides from where they would naturally cause acid rock drainage from weathering.
Mining removes and sequester sulfides from where they would naturally cause acid rock drainage from weathering.
In this way modern mining could in fact locally improve water quality where acid environments harmful to aquatic life might not naturally prevail.
In some cases acid rock drainage is a natural phenomenon.
Some misleading call it acid mine drainage as if it’s exclusive to mining but acid rock drainage only occurs where there are sulfide minerals and many mines excavate rocks that do not even contain these minerals.
In fact there are tens of thousands of naturally acid streams around the world draining from areas where there has never been mining and pyrite rich rocks naturally occur. In mining the potential to increase acid drainage is well understood now.
Excavation activity like mining and construction now put established engineering measures in place to ensure that the environment and human welfare is protected during mining and especially after mines are closed.

