Table of Contents
It is important to have Assay Laboratory standards over a range of grades. This alone may preclude the material being sampled from serving exclusively for the preparation of standard material. One of the standards must have as close to an absolute zero value as possible. At least three and if possible five other standards should be prepared. These standards should have the following distribution of grades:
- About half-way between the possible cutoff grade and zero.
- Near the average of all the mineralized material. (The following are useful if a potential cutoff can be determined and standard material of sufficiently high grade can be produced.)
- Slightly smaller that the possible cutoff grade.
- Slightly larger that the cutoff.
- Near the average grade above cutoff.
This distribution may need to be modified according to the availability of adequate standard material for each category. The most critical standards are the “blank” and those near the cutoff even if the cutoff is initially speculative.
Experience has shown that even by following the above guidelines about 30 to 40 percent of the candidate material will prove unacceptable and will be rejected. Prudent practice is to collect sufficient material to compensate for the anticipated rejection rate.
Acceptable Assay Lab Standard Material
If the material were perfectly homogeneous and the labs introduced no assay error, all the assays would be identical. No material is perfectly homogeneous and random (or inexplicable) errors do occur in the lab. Therefore, if ninety of the one hundred assay values are within ±5% of the grand mean calculated across all labs, the material in that lot is designated as an adequate standard. Because each lab handles fire assaying slightly differently, statistically significant differences among labs may occur. If three or more of the labs return values where ninety percent of the assays are within ±5% of the grand mean calculated using only samples from the labs showing the least variability, then the lot is declared to be sufficiently homogeneous to qualify as a tentative standard. One way analysis of variance can be performed to identify which labs differ both by mean and by within lab variability, though it is usually sufficient to calculate means and standard deviations by lab to make these identifications. It is easy to see that one lab has results that are considerably different from the others both in mean and standard deviation. Since the three labs agree quite closely the results from this fourth lab are suspect.
If only three labs meet the ±5% criterion a second batch of material should be divided from the remaining material in the lot and sent to just these labs for another round of assaying. If ninety percent of these values are within the ±5% limit, the material is accepted as a standard. For material with grades above 2 g/t, the acceptance limit is relaxed to ±8% to account for the increased intrinsic heterogeneity typically associated with higher grades. Whether this is justified may be determined from the sampling nomograph.
If any lot fails to meet the heterogeneity requirements, the material should be discarded and new candidate material should be obtained. The 5% rule is somewhat arbitrary. It assumes that sampling error contributes approximately 2% of the total error and 3 % is the result of the assaying process. At the discretion of the user and when there is difficulty finding sufficiently homogeneous material in a certain grade category, lots which consistently produce assays with a relative standard deviation of 6-8% may be designated as standards.
Procedures for Using Assay Standards
To monitor the stability of the assay process, the standard material is submitted and analyzed with batches of project samples. As discussed in the introduction, since the standard material is already fine, it cannot be inserted into the lab’s sample preparation stream. The lab must be instructed to insert standard samples into groups of project samples just prior to fire assay. The lab should not be made aware of the value of the standard submitted for inclusion with each batch of project samples. To make the standards as “blind” as possible to the lab, several different standards including the blank standard are assigned to batches with a systematic or stratified random sampling scheme.
A numbering system must be developed for the standard samples submitted with the project samples. The lab must not be able to identify the value of the standard from the numbering system, but the submitter must know by the number which standard was assayed. This numbering aspect is fraught with the possibility for generating errors. It is, therefore, very important that procedures for assigning samples numbers be thought through carefully, documented thoroughly, and followed exactly to avoid introducing still more error.
Depending on the amount of control required (which depends on the magnitude of potential losses from assay errors), a standard should be assigned to every 10 to 25 samples. Care should be taken to insure that the lab is assaying the standards with the project samples and not as a separate batch. This usually involves on-site inspection of the assay process at irregularly spaced intervals.
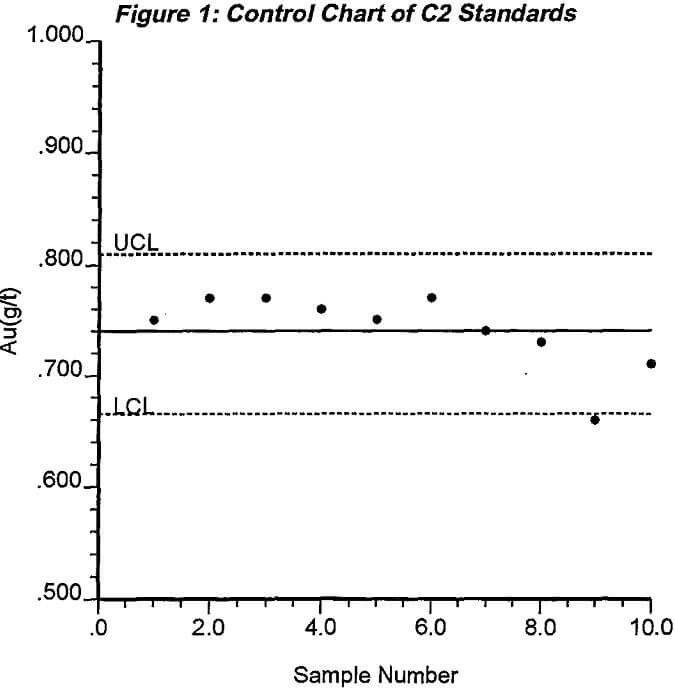
Process control charts should be used to monitor the lab performance with each standard. Process control charts are basically scatterplots of the assay values for a particular standard plotted as they are received. An example control chart for one standard assayed 10 different times is shown in Figure 1. The horizontal line in the middle of the chart shows the accepted value of the standard. The two lines parallel to the mid-line are at values 10% greater and 10% less than the accepted standard value. These are the control lines. The value of 10% is used since it is expected that 95% of the values will fall within two standard deviations of the true value if the process is in control. If the standard material shows more homogeneity, i.e., the relative standard deviation of the errors is less than 5%, then the control lines are drawn closer to the accepted value line. Assays of the standard that fall between the lines are designated as being in control and are an indication that the process is operating under control. If more than one assay in twenty falls outside the limits the process is said to be out of control. The extraordinary causes of such deviations should be identified and corrected before assaying any more samples. To be effective, the lag between when the assay of the standard is complete and when it is recorded on the control chart should be almost immediate. Otherwise, the process continues to operate in an out of control fashion jeopardizing the results from subsequent samples.
Steps to Improve Assay Results Quality
Standards must be incorporated in an overall program of process improvement to really be used effectively. It is not enough just to monitor and reject deficient results. The steps below list a broader view of how to improve the quality of assay results.
- Reject the idea that competition alone will somehow cause quality to improve. Assayers only have to be as good as their competitors not better.
- Look for value not the lowest price. Re-assay is likely to be costly. Pay for results not for effort.
- Establish an active involvement with one main lab and a second for check assaying. To choose this lab the following should be considered:
Are lab personnel experienced?
Are the personnel knowledgeable about both sample preparation and assay procedures?
Is the lab likely to be around for the long term?
Is feedback and involvement accepted or welcomed by the lab? - Work through a system of monitoring and process improvement with the lab. This usually involves the inclusion of standards as part of the stream of project assaying, and periodic assaying of runs of randomly selected established standards. It is an ongoing program that will not stop with the achievement of some arbitrary goal. Feedback to the lab and involvement in the assay process should continue for as long as business is conducted with that lab.
- Continually work with the lab to identify and reduce variability causing factors in the assay process. Meeting specifications does not result in improvement, only the status quo.
