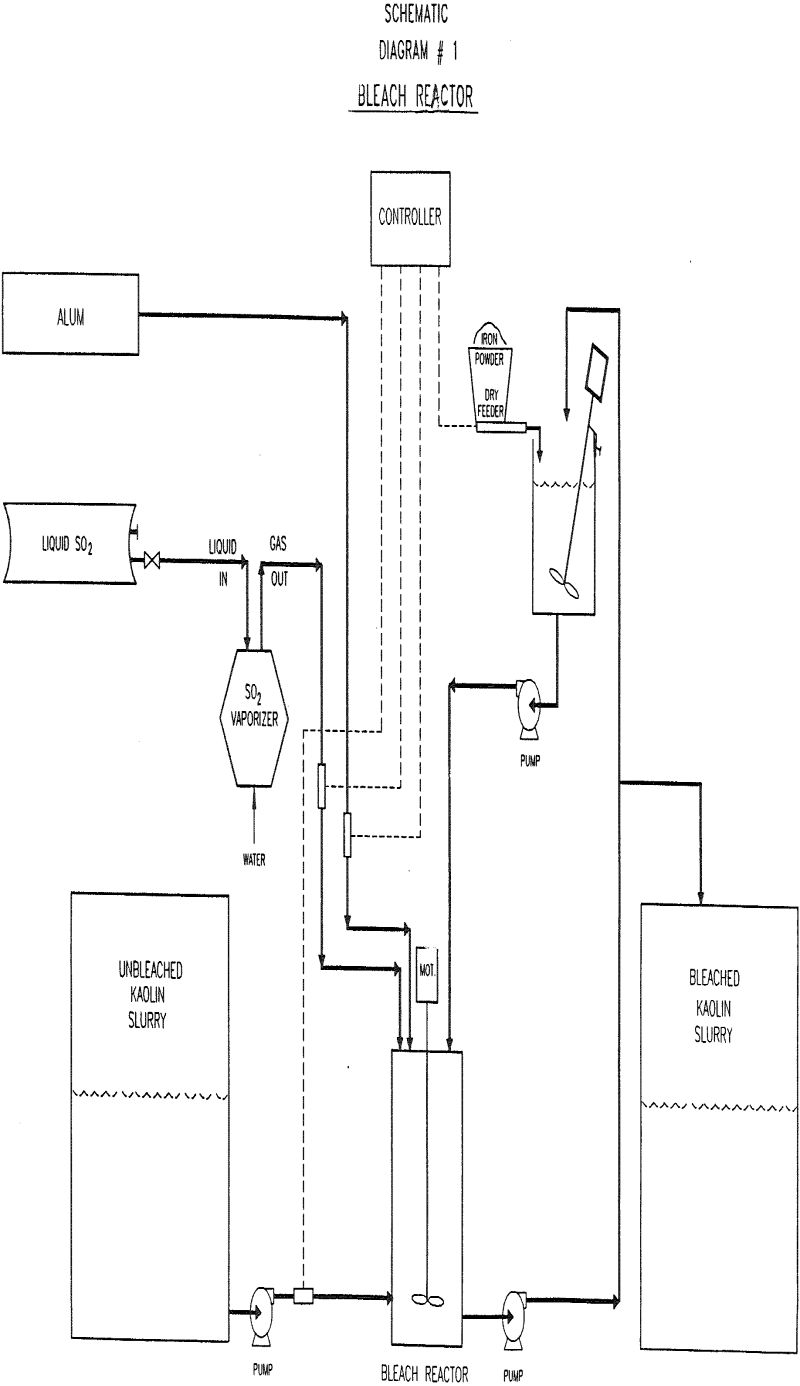
A process for producing reductive bleach in-situ in kaolin slurries has been developed. The bleach is formed by mixing an iron sponge powder (-325M) with slurry treated with SO2. The process has been successfully demonstrated in production, and applied to several grades. The color, brightness, and viscosity of the bleached product is equivalent to or better than that of product bleached with commercial sodium hydrosulfite. The tested products can be bleached at a cost, which is 38-54% of those associated with the use of commercial bleach.
Reduction bleaching is used to improve the brightness and color of the kaolin pigments sold to the paper and specialty industries. The bleach reduces the iron oxide associated with kaolin deposits to a colorless form by the following reaction.
Na2S2O42 + 6Fe+³ + 4 H2O → 2 NaHSO4 + 6Fe + 6H+
Unbleached kaolin slurries (20-30% solids) were bleached in the laboratory by adding iron powder and a source of sulfite ions. The average particle size of the iron powders tested ranged from 44 microns to 1500 microns. The sources of sulfite ions included either sodium sulfite, sodium bisulfite, sodium meta-bisulfite, or sulfur dioxide. When necessary, the slurry was adjusted to a pH value of 3 with sulfuric acid. In some experiments alum was added as the commercially available solution. Samples were allowed to react for times varying from 30 min to 16 hours.
During production trials, control samples were taken from the production bleach line in which liquid sodium hydrosulfite bleach, acid, and alum were mixed in the slurry with static mixers.
The bleach reaction rate is affected by the particle size of the iron powder. A sample treated with a 1500 micron iron powder was bleached from a brightness of 82.6 to a brightness of 84.5 after one hour of reaction time. The maximum brightness potential of 86.8 was measured after 16 hours.
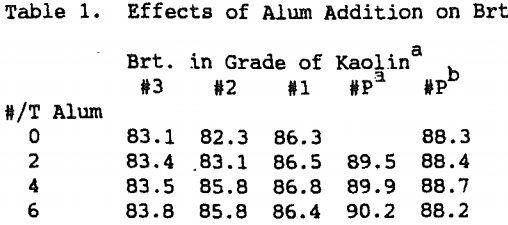
The optimum concentration of iron varied with the type of kaolin being processed. Products made from Tertiary crudes had demands of about 0.2 Kg/t (0.5 lb/t) whereas products made from Cretaceous crudes had demands between 0.5 and 0.75 Kg/t (1 -1.5 /t).
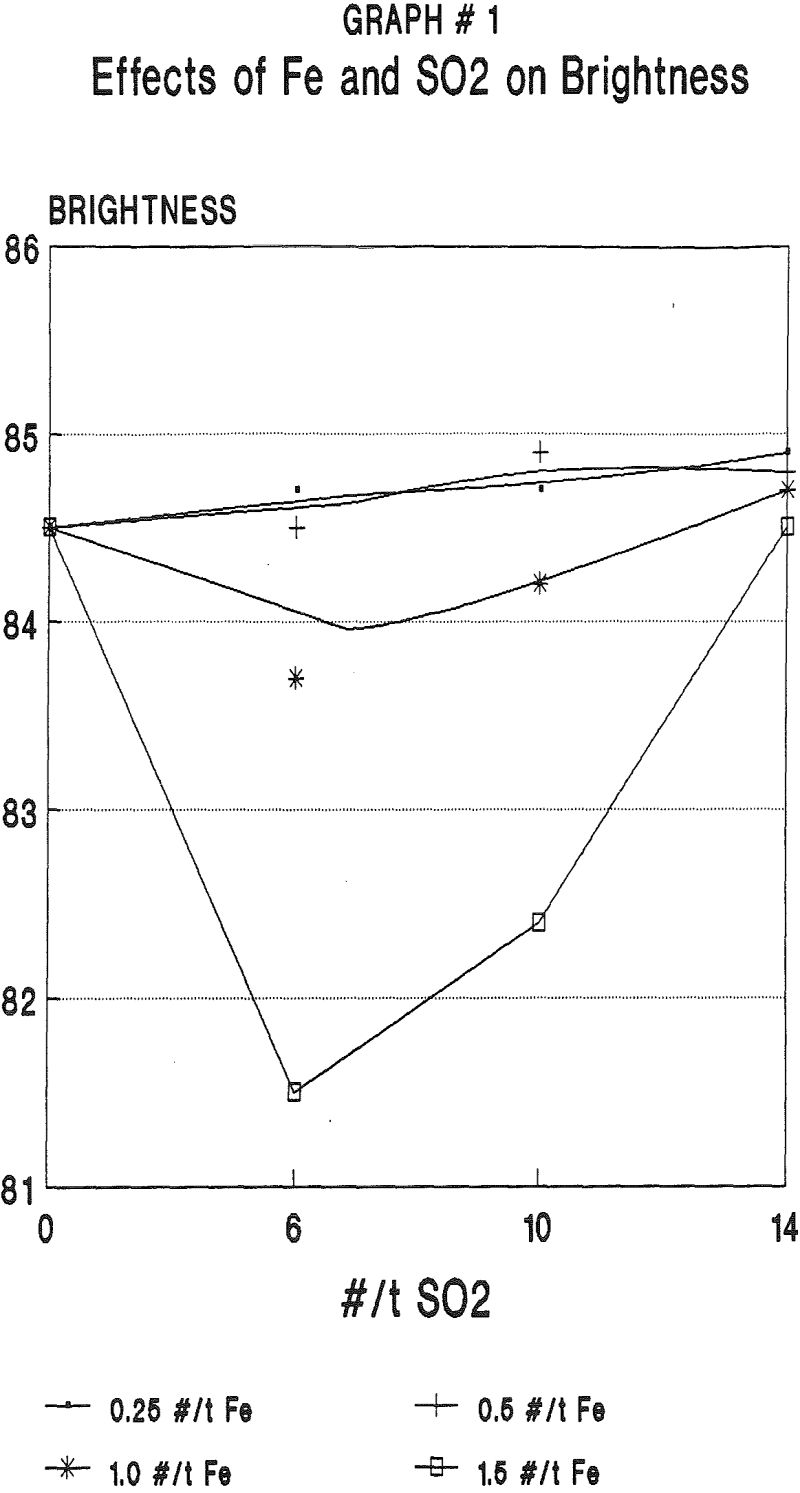
Although some bleaching was seen with sodium bisulfite and sodium sulfite, the maximum bleached brightnesses were seen using acidified sodium metabisulfite or sulfur dioxide. Sulfur dioxide was found to be superior in performance and cost to the other reagents.
Flocculation of the unbleached feed with alum or sulfuric acid was tested as a method of decreasing the levels of SO2 required by the in-situ process. Alum addition was found to decrease the level of SO2 required to obtain maximum brightness.
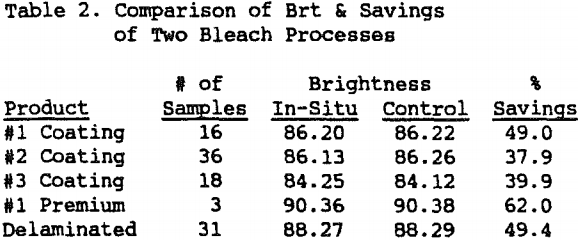
Five different product streams were successfully bleached in plant trials by the in-situ process using the demonstration unit. The products varied in brightness potential from 84.4 to 91.0
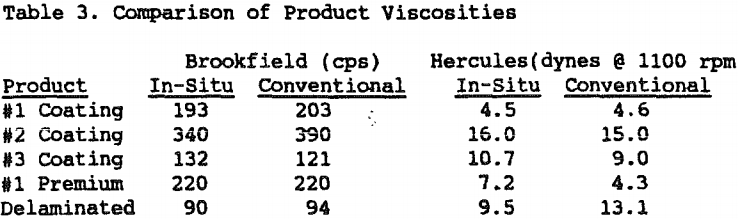
A 2 (80%-2 micron) coating clay was found to filter at an equal or slightly faster rate than the conventionally bleached product. Rates for the in-situ and conventionally bleached product were 9.9T/Hr and 8.9T/Hr, respectively.