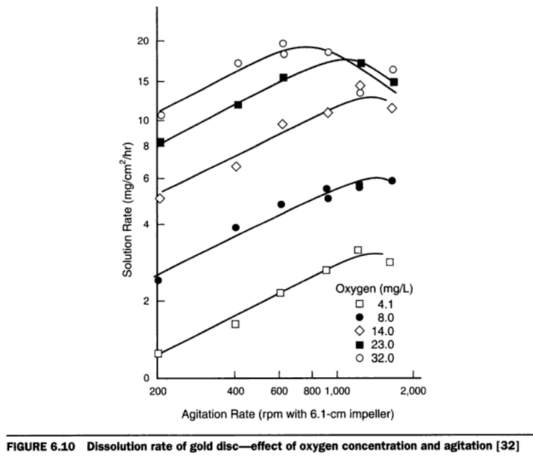
 In discussing the effect of oxygen on the gold dissolution, one can understand the use of oxygen or an oxidizing agent is essential for the dissolution of gold under normal conditions of cyanidation. Such oxidizing agents as sodium peroxide, potassium permanganate, bromine, chlorine have been used with more or less success in the past; but due to the cost of these reagents and the complications involved in handling them they have fallen into disuse. In addition, a greater understanding of the reactions involved in cyanidation and a more complete knowledge of the parts played by various undesirable constituents in ores, have shown that adequate aeration under the right conditions will often give as good results as chemical oxidizers.
In discussing the effect of oxygen on the gold dissolution, one can understand the use of oxygen or an oxidizing agent is essential for the dissolution of gold under normal conditions of cyanidation. Such oxidizing agents as sodium peroxide, potassium permanganate, bromine, chlorine have been used with more or less success in the past; but due to the cost of these reagents and the complications involved in handling them they have fallen into disuse. In addition, a greater understanding of the reactions involved in cyanidation and a more complete knowledge of the parts played by various undesirable constituents in ores, have shown that adequate aeration under the right conditions will often give as good results as chemical oxidizers.
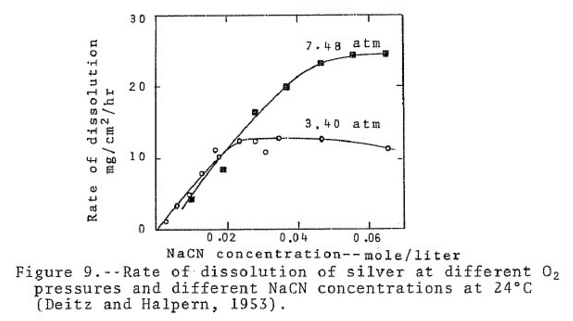
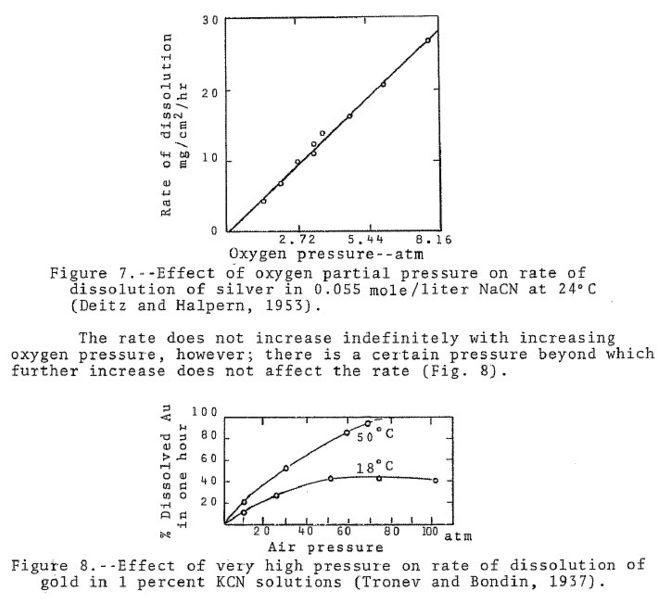
Cyanide researches determined the rate of dissolution of gold in 0.10 per cent NaCN using nitrogen, oxygen and mixtures of nitrogen and oxygen. The tests were conducted on 100 ml. volumes of cyanide solution, at 25 ‘C, and with equal volumes of gas for each test. Throughout each test the rate of dissolution was uniform except when oxygen alone was used. In the latter case the gold dissolved rapidly for the first half hour and then slackened off considerably. The experimenters attributed this to polarization. The results showing the rate of dissolution for the first half hour of each test are shown in the Table below. From these results it may be noted that the rate of gold dissolution was directly proportional to the oxygen content of the gas used. From this, the experimenters suggest that the rate of dissolution of gold in cyanide solutions is directly proportional to the partial pressure of oxygen.
| Effect Of Oxygen On Rate Of Gold Dissolution | |
|
Oxygen % |
Rate of Dissolution mg/sq. cm/hour |
|
0 |
0.04 |
|
9.6 |
1.03 |
|
20.9 |
2.36 |
|
60.1 |
7.62 |
|
99.5 |
12.62 |
https://www.academia.edu/292086/Kinetics_and_Mechanism_of_Gold_and_Silver_Dissolution_In_Cyanide_Solution