Gold and elements of the platinum group metals have been found in several types of deposits around the world. Sometimes, deposits have been worked for their cobble and gravel components and these places have been set up to recover heavy minerals (mainly as black sands) from their host rock. These minerals carry precious metals and they can be recovered by gravimetric devices such as shaking tables or centrifugal concentrators. Most the time, quartz, garnet, pyrite, magnetite, hematite, zircon and monazite are found in these deposits and they can be concentrated by any gravimetric equipment. Try to understand the genesis of each deposit and the minerals present is a very important aspect that must be considered in the extraction process to be used to recover gold.
Mineralogy can be influenced by gold particle size, non-valuable minerals, type of non-ferrous metals, and existence of disseminated or agglomerated gold particles. The factors mentioned play a very important role in the recovery of gold. One condition very important is the particle size because the selection of one or multiple processes is conditioned to the particle size.
Gold Occurrence
Occasionally large accumulations of native gold (metallic gold) occur, but usually gold is founded as very small grains. These grains occur between mineral grain boundries as inclusions within minerals. Gold is widely distributed in the Earth’s at around of 0.004 g/t. Hydrothermal ore deposits of gold occur in metamorphic rocks and igneous rocks; alluvial deposits and placer deposits originate from these sources.
Groundwater solutions (meteoric water), and/or solutions emanating from a cooling magma (hydrothermal fluids), and/or fluids ejected from compressing sediments (pore water) penetrate along fractures and tiny pore spaces between mineral grains in the rock. Under certain conditions these solutions, which contain different compounds, may dissolve, deposit other minerals, or alter the rock-forming minerals. Leaching, or the decomposition, dissolution and removal of soluble minerals from the rocks may be caused by hydrothermal or meteoric solutions. The dissolved mineral products may be dispersed or redeposited elsewhere as the same or different minerals, leaving often greater concentrations. Relative content of gold may be increased by the removal of originally associated minerals, leaving an enriched residual gold deposit.
The primary source of gold is usually igneous rocks. Pressure and temperature within the earth increase gradually with distance below the surface. At depths of several tens of miles the material which makes up the earth’s crust (depending upon its composition) may become partially molten. This molten material is called magma, and is normally less dense than the overlying solid rock. As the magma is subjected to tectonic forces, it may be squeezed upward along weak zones in the crust. This process by which magma penetrates the crustal rock is called Intrusion. The intrusive magma which has cooled and solidified is known as igneous rock. Common examples of igneous rocks are granite, granodiorite, and diorite.
A gold deposit usually has a secondary form of enrichment that can be either chemical or physical processes like erosion or solution or more generally metamorphism, which concentrates the gold in sulfide minerals or quartz. Decomposition and disintegration of rocks at or near the surface by physical and chemical processes is called weathering. The products of weathering are normally carried off by erosion. Water percolating into the ground dissolves some minerals from the rocks and forms acidic or basic solutions which further attack the rock and break it down chemically.
The distribution of gold seems to validate the theory that gold was carried toward the earth’s surface from great depths by geologic activity, perhaps with other metals as a solid solution within molten rock. After this solid solution cooled, its gold content was spread through such a great volume of rock that large fragments were unusual; this theory explains why much of the world’s gold is in small, often microscopic particles. The theory also explains why small amounts of gold are widespread in all igneous rocks.
- Native Gold
Since gold is relatively unreactive, it can be found uncombined, free or native state. All the three last words are referred to metallic gold in nature. For this reason, gold was found and used many years BC. Perhaps, this is the easiest way to recover gold, but unfortunately, this type of gold is scarce. Native gold rarely occurs in its cubic crystal form and can be founded in rounded particles called nuggets. Gold does not display cleavage on breakage. There are many terms to describe the various forms of native gold: sponge gold, flakey gold, grain gold, foil gold, moss gold and tree gold.
Under this form, gold has a high purity (normally more than 90%). Also in this form gold is a very heavy metal with a specific gravity is the range 19-19.3. This characteristic has been used for many years in gravimetric devices to recover gold. Gold is obtained by two principal mining methods; placer and vein mining, and also as a by product of the mining of other metals. Placer mining is used when the metal is found in unconsolidated deposits of sand and gravel from which gold can be easily separated due to its high density. The sand and gravel are suspended in moving water; the much heavier metal sinks to the bottom and is separated by hand. The simplest method, called panning, is to swirl the mixture in a pan rapidly enough to carry the water and most of the gravel and sand over the edge while the gold remains on the bottom. Panning is the classic method used by the old miners and is immortalized in story, art, and song.
The predominant occurrence of gold is as native metal, often alloyed with up to 15 % silver. Other gold minerals include alloys with tellurium, selenium, bismuth mercury, copper, iron, rhodium, and platinum. There are no common naturally occurring gold oxides, silicates carbonates, sulphates or sulphides. Therefore, gold generally occurs in a mineral form different to most other elements which allows selective gold extraction from other mineral mixtures.
In addition to its softness, it is both the most malleable and most ductile of all elements. This means that it can be hammered into extremely thin sheets (approaching a small number of atoms) and can be drawn into extremely fine wire. Gold in the form of very thin sheets, called gold leaf, has many decorative uses. Elemental gold is an excellent conductor of electricity and heat, surpassed only by the other members of group 1B, copper and silver. Gold usually forms compounds by giving up either one of three of its valence electrons. It is commonly alloyed with other metals, as in jewelry, in proportions that yield desired hardiness and colors, (see metal section). An alloy of gold, silver, and copper, in which the amounts of silver predominates, is called green gold. An alloy of the same three elements in which copper predominates is called red gold. An alloy of gold and nickel is called white gold. The purity of alloyed gold is expressed by the karat system, where the percent of gold by weight is given as a fraction of 24. Therefore, pure gold is 24 karat, whereas 18 karat gold is 18/24, or 75%, gold by weight.
- Electrum
The natural gold source called electrum is an alloy of gold and silver, with trace amounts of other metals, giving electrum a warm, moonlight-tinged hue of pale to bright yellow. Electrum has been found in Turkey. The name is a Latin form of the Greek word elektron that was mentioned in Homer’s eighth-century BC epic, the Odyssey. The content is variable and can be as high as 70-90% and the proportion is different according to the geographical location. Thus is better to consider more than one type of electrum according to the gold and silver proportion.
- Calaverite
Calaverite is one of the names for gold telluride, the natural combination of gold and tellurium. Calaverite is named for Calaveras, California where was identified for first time. The mineral occurs in veins with the other tellurides associated with gold. Calaverite and other associated gold and silver telluride minerals are important sources of silver and gold in the mines, at Cripple Creek and Kalgoorlie, W. Australia. Calaverite and krennerite are minerals containing 40% gold, and sylvanite with 25% gold. The other elements are present in variable proportion and silver is the main element of this group. Its color may range from a silvery white to a brassy yellow.


Electrum Calaverite
- Unusual gold occurrences
Gold can be found associated with arsenic and antimony. In the first case, arsenopyrite has gold particles, and in the second one, stibnite is the gold carrier. Sometime, the gold recovery from these minerals is very complex due to gold exist in very fine particles that can not be liberated by simple processes of crushing and grinding. The cyanidation process can be painful with these minerals and will be necessary to consider preliminary processes oriented to make less complex the gold recovery.
Sometimes, carbonaceous compounds are associated with gold. Most the time the gold is present in very tiny particles and the recovery process is difficult. Carlin Mine is the main example of this type of complexity.
There is an occurrence of gold-nickel ore from Alistos, Mexico. It consists of two types, first one, gold-niccolite, containing minor amounts of gersdorffite and maucherite; second one, gold-millerite, with associated pentlandite, gersdorffite, and violarite. The mineral association of native gold with niccolite and primary millerite, is considered unusual. The ore is believed to have originated from solutions which produced alteration in a peridotite stock.
Gold Sources
There are different gold sources on earth. They are influenced by geological aspects and its economical treatment is many times complex. A common classification considers placers, vein ores, oxidized ores, polymetallic sulphides, and special sources. Each of these types of gold sources has especial mineralogy which play an important role in their metallurgical treatment.
- Placers
Gold deposits commonly are found as primary lode deposits in the bedrock or as superficial placer deposits resulting from erosional reworking of bedrock sources. Lode gold deposits are formed by hydrothermal fluids and commonly take the form of veins and sometimes are associated with arsenic. Placer deposits are formed by selective concentration of heavy minerals with specific gravity greater than 2.7. These minerals are found in eroded bedrock deposits within river systems and shorelines. To survive erosional environment, these minerals must resist chemical attack. It’s common to find in placers gold, rutile, zircon, monazite, and magnetite. Placer deposits are quite variable in size, ranging from 10s to 1000s of meters in width, and with pay streaks of 1 to 10s of meters thickness, occurring above bedrock.
Placer deposits have been responsible for near 70% of gold production in the world, as well as major sources of other minerals. Much of this gold, with the exception of the giant Witwatersrand deposits in Africa has been retrieved from preglacial gravels by washing operations. Many classic gold rushes such as Klondike were focused ion rich placers in active river systems. Unfortunately, these types of deposits are small by current mining standards and environmental concerns now restrict extraction from many rivers.
Placer deposits may be categorized according to their age, size, means of formation, mineralogy, physical and chemical characteristics, and geomorphological forms. The most common classification is based on deposit geomorphology that comprises residual, eluvial, stream/alluvial, eolian, glacial stream and beach placers. There are three variables very important in placer formation, first tectonic regime, second composition of the substrate, and third the climate. The last one has a very strong control in the process formation. The gold content in placer ores is usually low compared with the associated primary hard rock deposit from which they were formed. However, due to the easy operation and low costs, placers are often commercially significant and may be the forerunner to further underground mining.
Gold particles once released by erosion from their bedrock, undergo physical attrition and deformation to a degree proportional to the distance of transport. Metallic gold is very malleable and can form several contrasting physical characteristics dependent on the original style of mineralization, the mode and distance of transportation, and any subsequent weathering. Grain characteristics are described by flatness, roundness, shape, and surface texture. Primary gold can be found as platy, square and irregular shapes. During the secondary process formation, gold particles round edges and smooth protrusions, even can be possible to observe a new flattening process.
It is common to find rimming of gold grains. Rims can be 5 to 18 µm thick. This phenomenon is the result of the different solubility of gold and silver in the low temperature weathering environment that leads to dissolution of silver leaving a gold metallic residue. These rims are characterized for containing gold particles of high purity.
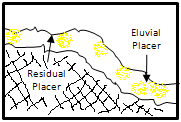
Residual placers are accumulated over the main rock source. This formation is due to decomposition and elimination of lighter materials than the rock. Sometime, these formations are oriented to eroded veins.
Eluvial placers are found in sloped ways and can contain minerals liberated from the closer rocks. Heavy minerals are concentrated on the surface and lighter minerals are dissolved or transported. These actions produce a partial concentration due to a volume reduction. Obviously, deposits with economical interest, the source rock must have an important content of gold.
Alluvial placers have been a very important source of gold and the first miners worked these deposits. The gold extraction is usually easy and they have been the reason of passionate gold extraction in California and El Yukon two centuries ago.
Beach placers are formed by waves. The formation of zones with heavy minerals is due to movements in two directions, towards the beach and to the ocean. In this way, waves can classify light and heavy minerals in the coast. A very important factor is the sea level because the exposed area is variable. This phenomenon occurs in Australia and Africa where there are important mining operations.
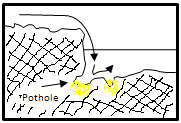
Placers Concentration in potholes
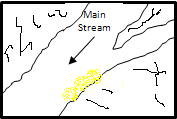
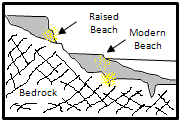
Effect of water flow Beach placer formation
When two streams are joining, the water flow promotes points with gold content. In this case one stream has higher water flow than the other. The degree of gold liberation and the surface chemical properties of placer gold are important in the effectiveness of gravity concentration and amalgamation. As most gangue minerals are lighter than gold, unliberated gold grains are recovered less efficiently by gravity concentration. Detailed surface chemical data on gold grains in placer deposits are not abundant, however, it is known that sulphur and hydrocarbon adsorption can occur and the presence of impurities in the gold significantly affects amalgamation.
- Leachable ores
This ore group is also called free-milling ores and is formed by deposits with gold contents higher than 5 g/t and the process extraction recovery is as high as 90%. Normally, the ore treatment process follows crushing, comminution, gravimetry and cyanidation. This kind of ores are found in placers, quartz vein gold ores, oxidized ores, silver-rich ores, copper sulphide ores, and some iron sulphide and arsenic sulphide ores.
An aspect very important is to perform mineralogical studies of gold behavior in various ores and metallurgical products in order to classify the ore and address issues and problems related to gold ore processing. It is widely used as a predictive and trouble-shooting tool in gold ore processing, and provides useful information on process selection, flowsheet development, recovery improvement, and reagent consumption optimization.
The gold behavior of the several ore types can be summarized as follows: gold in placers, quartz vein gold ores, and oxidized ores are easily liberated, and can be recovered by gravity, flotation, and cyanidation; gold in copper sulphide ores is often coarse-grained and associated with copper minerals, and can be recovered into a copper concentrate by flotation; gold in silver-rich ores is often present as electrum or associated with silver minerals, which can be recovered by gravity, flotation, and/or direct cyanide leaching. The problem in recovering gold from silver-rich ores is that the greater reactivity of silver can influence the metallurgical performance of gold in flotation, leaching, and recovery processes by the formation of silver sulphide or silver sulphate coatings.
One good example of leachable ore is the Witwatersrand deposit in South Africa. This deposit is the bigger auriferous concentration in the world. Since was discovered in 1886, the estimated gold production is near of 45,000 tonnes, which represents approximately 40% of total production of gold in the world. In 1983 some mined zones had a gold content of 10 g/t. Nowadays the gold content is lower. This type of deposits consists of lithified conglomerates which contain rounded pebbles of quartz in a matrix of pyrite, fine quartz, uraninite, and gold. There are have been some hypothesis that tried to establish an association between gold and uranium oriented to determine if uraninite is a chemical precipitated or not.
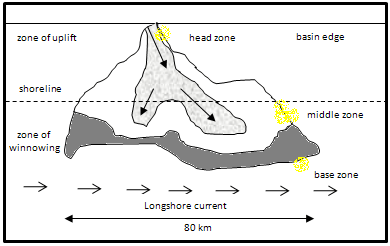
Figure shows a Witwatersrand fan formation. Gold has a higher concentration in the middle zone.
- Weathered ores
Weathered ores or oxidized ores are the product of sulphide destruction by weathering under oxidizing conditions. Pyrite is the most significant source of oxidized sulphur and indirectly, hydrogen ions, in a sulphide.
An oxidized ore is defined as one in which the ore material has been oxidized or weathered, possibly in a zone that is atypical of the primary sulphide deposit, and for which some especial processing may be required bearing rock volume undergoing weathering. As these components are interrelated, oxidation of pyrite is important to produce altered sulphides.
Oxidation of pyrite comprises a series of processes that creates oxidized iron minerals whose generation affect the oxidation process of other metallic sulphides. In general, there is ferrous, ferric and sulphate ions tend to handle the oxidation process. In this way, hydroxyl-sulphates are generated constantly and part of these compounds will be deposited in parts of the rock during the formation process creating conglomerates of particles with high permeability. Under this fact, some leaching process are favored due to the leaching agent can penetrate into the particles. This is the success of many heap leaching operations that form the heap without a previous crushing stage.
The mineralogy of the host rock plays a very significant geochemical role in the development of gangue and ore mineral assemblages occurring in the oxidation zone. In general, host rock such as silicates and oxides are a very important part in the oxidized environment due to they have exchangeable cations able to take hydrogen. If the quantity of reactive silicates and carbonates is high, the ability to neutralize sulphate bearing solutions is enormous.
Weathered ores can be formed by hydrothermal alternation. In this case, alteration and mineralization are produced in two stages. First, magmatically vapours ascending along fractures are absorbed into groundwater and form an acid fluid. Second, the acid fluid leaches the host rock producing an porous rock, sometimes called vughy silica. These acids assemblages can be associated with clayed zones. Early advanced argillic alteration in the area creates additional permeability in fracture zones. Intense acid leaching created pathways for metal bearing fluids and produced the open space vughs which host metal sulphides and Au.
Try to understand the environment of formation is a key aspect of the economical and metallurgical performance of these deposits. Many deposits are characterized for having a reddish color due to the presence of hematite. The generation of red hematite during oxidation of supergene sulphides is explained by the oxidation of pyrite.
Ore types of oxidized gold ores have gold contents as high as 10 g/t and an agitated leaching process is a good and attractive alternative due to the leaching is high and consequently the gold extraction is high, typically more than 90%. However, sometimes there is a potential problem, the presence of high amounts of oxidized copper minerals. The problem is related to the high cyanidation consumption and low gold recoveries.
The flotation process is other alternative, but the excessive floatability of fine material is an issue to be considered. The presence of clay is other potential problem due to this material consumes reagents and modifies the rheological properties of the slurry. When the presence of fine particles is not problematic, silver and gold will be recovered.
- Silver-Gold ores
Some gold deposits can have silver contents as high as 8 oz/t and gold contents are ranging 1-2 g/t. In this case the presence of silver sulphide minerals is important and they are associated with gold. Consider the high proportion of silver the usual recovery process is flotation due to silver minerals has a high rate flotation. To include a gravimetric device ahead of flotation is other possibility.
- Iron sulphides minerals
It is well known that iron sulphide minerals, arsenopyrite, pyrite and pyrrhotite are host minerals for gold. Most the time, gold can be extracted by cyanidation and can be categorized as not refractory. It’s possible to make an iron sulphide-gold concentrate and perform a selective separation between the iron mineral hosting gold and non- valuable iron sulphide minerals. This separation is practiced by flotation under different conditions that are base on reduction-oxidation conditions.
The presence of arsenopyrite can be an issue if the process selected is flotation and cyanidation due to the high consumption of oxygen. In this case special pre-treatments before cyanidation can be included in order to minimize this problem. There is other potential problem when the final product is obtained by flotation and must be commercialized. The problem is the high content of arsenic. If the mineral is refractory to cyanidation, the problem is more serious.
If the ore body has pyrrhotite, the cyanidation process can be problematic due to this iron sulphide mineral takes very easy the oxygen from the environment. The leaching process is not efficient even at cyanide consumptions.
- Base metal sulphide minerals
Copper and polymetallic sulphides deposits contain gold in several forms, free or as fine particles hosted in iron sulphides. In general, gold associated with a particular base metal is recovered by flotation with the mineral, but sometimes when gold is hosted by pyrite appear some problems to the process. Basically, coarse free gold can be recovered by gravimetric devices ahead of flotation circuit. Fine gold must recovered in a copper or copper-lead concentrate, but never into the zinc flotation circuit because the extractive process of copper and lead concentrate includes the recovery of precious metals. The treatment of zinc concentrates is not a good option for precious metals. Base metal concentrates receives an extra pay for gold and silver. Then is attractive try to concentrate precious metals into copper and lead concentrates.
Sometimes, gold can be hosted by pyrite and its recovery will be achieved after recovering copper, lead and zinc. In this case, a pyrite concentrate with gold content will be obtained and the possibility of including a cyanidation process is attractive.
- Gold tellurides
Ore mineralogy and the deportment of gold in various forms and sizes as well as preferential associations of gold with other minerals are important factors in the application of recovery processes to tellurides minerals. In some mines, telluride minerals forms compounds with mercury, silver, lead and small amounts of nickel. The main tellurides are Calaverite, sylvanite, krennerite, petzite and nagyagite. These minerals can be recovered by flotation and pyrite is a common contaminant. The material can be refractory to leaching and is necessary to consider an alternative process previous to cyanidation. Roasting is an option, but there are losses, gold deposition occurs on internal walls of the roaster. Basically, refractory occur is due to some of gold is finely disseminated in the host rock or in solid solution with pyrite.
- Non-sulphide gangue
Many gangue minerals can host native gold and was noted that the gold does not replace the host mineral. Apparently, gold is readily liberated by comminution operations and then can be recovered by gravimetry, flotation and cyanidation. The process is influenced by the particle size due to the efficiency of the processes mentioned are influenced by the gold particle size. If there is particular mineral association, it wil be advantageous to recover that specific mineral. The type of gangue, for example quartz, silicates, carbonates and iron oxides have an effect on the recovery process due to modify the slurry properties. A particular case can be considered when the amount of clay minerals is high. Normally, the latter is a critical case.
- Carbonaceous material
Carbon can be present as carbonate mineral and inorganic material. The last one ranges from graphite, which is a poison from a treatment consideration, to the named carbonaceous matter that has near of eighty percent of carbon and consists of active element carbon, hydrocarbons and organic acids. This type of carbon is problem when the ore must be treated by cyanidation due to cause the called effect of pre-robbing. This phenomenon consists in absorbs the free cyanide and gold cyanide complex from cyanide leach solutions. This situation is found in Witwatersrand ores and there is association between organic matter, uraninite and gold. Try to treat this ore is complex and needs more than one process in order to recover gold.
- Arsenic minerals
Basically the metallurgy of gold is related to three types of arsenic minerals: arsenopyrite, orpiment and realgar. The first one is the most common and gold can be present as fine particles. The last two minerals are found in minor proportion and can be found in weathered ore deposits.
- Non-ore material
Several non-ore material can enter to the extraction process and creates a negative effect on gold recovery. Some of them are ferrous wear debris, vegetation, metallic pieces from mining equipment, rubber and lubricants. The first group is derived from crushers and comminution equipment. Iron oxides are deposit on gold surfaces and affect the recovery a recovery process like flotation due to modify the polarity required to attach gold particles on bubbles. Cyanidation is affected by this situation.
Vegetation enters a plant feed when treating near surface ore or tailings dumps, material from underground operations. This material affects flotation and cyanidation. If this material is in contact with alkaline solutions such as pH 9.0, will be liberated water soluble organic compounds. Some of these products produce frothing agents that creates overfrothing.
