Table of Contents
Carbon strip circuits present a particularly challenging scale inhibition problem. Many of the factors that tend to promote scaling are present in these systems. For example, carbon strip circuits are run at high temperatures, high pH levels, and require long batch cycle times. Tortuous paths are encountered by the strip solution circulating through heat exchangers, pipes, and elbows, causing transient pressure drops throughout the system. Severe conditions such as these cause the water to possess a high scaling potential and may lead to the breakdown of some of common types of scale inhibitors.
Formation of Scale
Natural waters contain dissolved minerals and gasses which promote the scaling process. One of the contained gasses, carbon dioxide (CO2), is an important factor in scale deposition. Carbon dioxide dissolves in water to yield carbonic acid according to the following reaction:
CO2 + H2O → H2CO3 (carbonic acid)
Carbonic acid rapidly dissociates to yield bicarbonate and carbonate ions depending on the pH of the system:
H2CO3 → H+ + HCO3-(pH range 6-10)
HCO3- → H+ + CO3= (pH range > 9)
In gold cyanidation processes, lime is added to raise the pH of the mill solution to 10 or above to promote cyanidation of the gold and to eliminate the possibility of hydrogen cyanide (HCN) formation. Lime contributes both hydroxyl ions (OH-) and calcium ions (Ca++) to the solution. The normal scale forming reactions in mill water systems were described as:
HCO3- + OH- → CO3= + H2O
Ca++ + CO3= → CaCO3
Factor Affecting Scale Deposition
Solubility tables, the solubility of calcium carbonate in carbon-dioxide free water is only 13 mg/dm³ (13 mg/L) at 25°C (77° F). Scale deposition occurs when the concentration of calcium and carbonate ions in the solution exceeds the solubility of calcium carbonate. This occurs, in most cases, when the water has undergone some chemical or physical change. Some typical changes that might occur that lead to scale deposition are discussed below.
Temperature
An increase in temperature can greatly increase the tendency to form calcium carbonate scale. Studies have indicated that calcium carbonate is less soluble at higher temperatures. Hence, high temperatures promote scaling. For this reason, scale is often noticed on heat exchangers, autoclaves and other high temperature surfaces.
Pressure
A decrease in pressure may lead to an increase in the tendency to form calcium carbonate scale. Work has shown that scale is more soluble at high pressures. Hence, scale is likely to be prevalent in pump suction lines, elbows, baffles, and other areas that produce turbulence and subsequent pressure drops.
Change in pH
Calcium carbonate scale is pH dependent and tends to form more readily at high pH. Any event that causes an increase in pH, such as the introduction of lime or caustic, would tend to initiate scale formation. Since most gold recovery operations are run at a pH above 10, the tendency to form calcium carbonate scale is always great.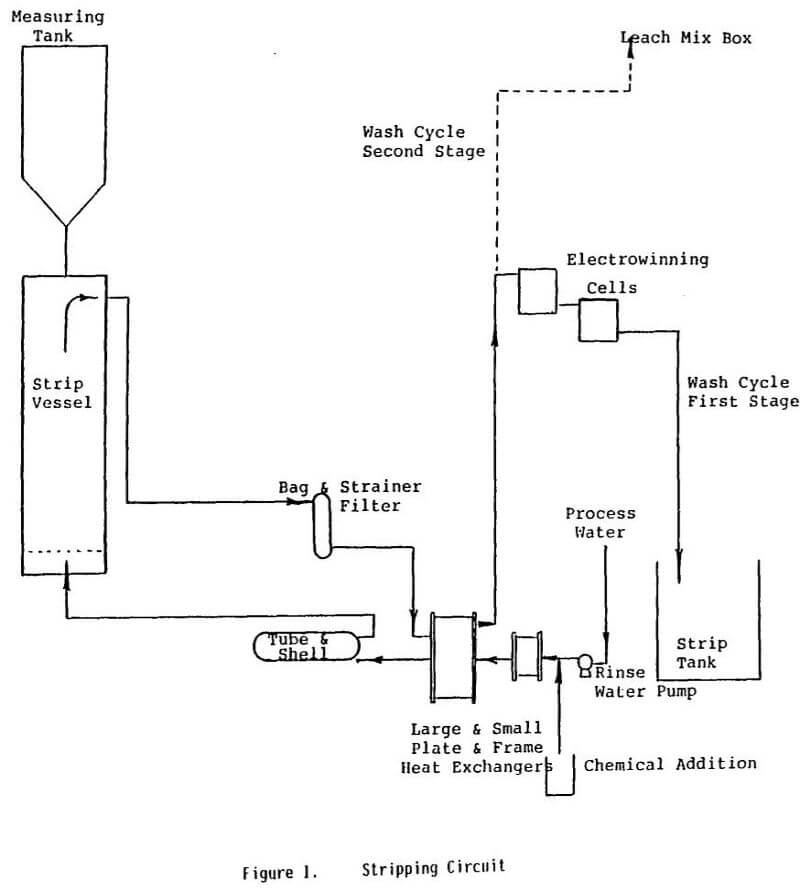
Use Scale Inhibitiors to Prevent Calcium Carbonate Scale
Many products are commercially available to inhibit the formation of calcium carbonate scale. Some of the more effective types include phosphonates, as well as acrylate and maleate-based polymers and copolymers.
Many of these products function by means of threshold inhibition mechanisms. The “threshold effect” is the prevention of precipitation of scale from supersaturated solutions by the addition of very small, sub-stoichiometric amounts of scale inhibitors. In this manner, several parts-per-million of scale inhibitor can prevent the precipitation of several hundred parts per million of calcium minerals. This was described as being accomplished by means of absorption of the inhibitor into the scale crystal lattice thus distorting the morphology, and adsorbtion of the inhibitor onto the crystal surface thus obstructing growth of the crystal.
Another means of scale control is by the use of dispersants. Dispersants function by attaching to the surface of mature scale particles and function by a charge repulsion mechanism.
A third means of scale inhibition is chelation, sometimes referred to as sequestration.
Chelation Solves the Problem
A substantial improvement in system operation was realized with the use of a proprietary antiscalant consisting of a specially designed chelating agent and organic dispersant. This product effectively sequestered the calcium in the strip solution and rendered it nonreactive, thus reducing further scale deposition. The organic dispersant in the product kept previously formed scale crystals free flowing. The scale deposits that were encountered were soft and it became possible to acid wash the plate and frame heat exchanger in place. This greatly reduced system down-time. This type of formulation also has the ability to clean from metal surfaces scale that was previously deposited. Thus, further minimizing the need for acid cleaning.
Reaction of the metal with the chelating agent may greatly alter the reactivity of the metal toward precipitating ions. In this manner, the reaction of a chelating agent with calcium ions in solution will prevent the calcium from reacting with carbonate or sulfate to form calcium carbonate or calcium sulfate. Since the complex is a very strong one, it is unaffected by temperature, pressure drops, high pH or long batch times. Hence this is the ideal scale inhibition chemistry to apply to carbon strip circuits.

