Table of Contents
The high reactivity of chlorine as an oxidizing agent in leaching ore has long been recognized, but chlorine has not been extensively used because of high costs and because of the corrosiveness of acid chlorine solutions. As early as 1848, the use of aqueous chlorine solution was proposed for extracting values from gold ores, and around the turn of the century, patents were granted for processing copper and zinc ores with electrolytically produced chlorine. Cheaper chlorine relative to present metals production costs and improved availability of corrosion-resistant materials for equipment fabrication may make the use of chlorine more attractive for hydrometallurgical applications.
A literature survey indicated that copper sulfide minerals and pyrite are readily dissolved by aqueous chlorine, but the amount of chlorine required, and the chemical reactions involved were not clearly defined. Therefore, an investigation was initiated to answer these questions. Chlorine leaching of copper sulfide minerals was the primary concern of this study, but pyrite also was included because of its common association with copper sulfides.
In this study, sodium hypochlorite was used as the source of chlorine. Sodium hypochlorite can be made by dissolving chlorine in a caustic solution, and the reaction is reversed with the evolution of chlorine when acid is added to the system. The equilibrium that exists in a neutral solution is represented by the following equation:
Cl2(aq) + H2O ↔ HClO + H+ + Cl-……………………………………………………..(1)
The concentration of dissolved chlorine is defined by the equilibrium expression
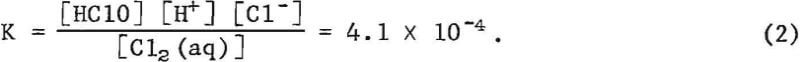
At low pH values, dissolved chlorine predominates in the solution unless the chloride ion concentration is low, and then hypochlorous acid is the pre-dominant species. Hypochlorous acid dissociates as the pH increases, and at pH values of 7.4 and above, hypochlorous ions (ClO-) predominate. These reactions are reversible and the distribution of chlorine among the various species is pH dependent.
Because the various species are so closely related, it is immaterial whether a chlorine-based process is referred to as a chlorine process or a hypochlorite process; one may be the reagent actually added, while the other may be the actual reacting species.
At the low pH (about 2.0) of the leaching solution used in this study, chlorine gas was evolved when sodium hypochlorite was added and dissolved chlorine was the active oxidizing agent. The use of sodium hypochlorite provided a convenient method for adding a known amount of the oxidant.
Material Equipment and Procedure
The selected sulfide minerals used in this investigation were covellite, chalcopyrite, chalcocite, bornite, and pyrite. The chalcopyrite sample was a flotation concentrate which was further upgraded by additional flotation with pyrite depression. The other minerals were specimen-grade samples and were upgraded by hand selection. Each sample was crushed and wet screened to obtain minus 100- plus 325-mesh material. The sized fractions were further upgraded by isodynamic magnetic separation. Chemical analyses of the minerals samples are given in table 1.
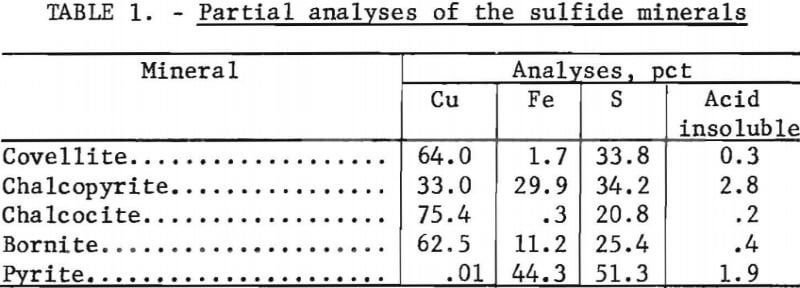
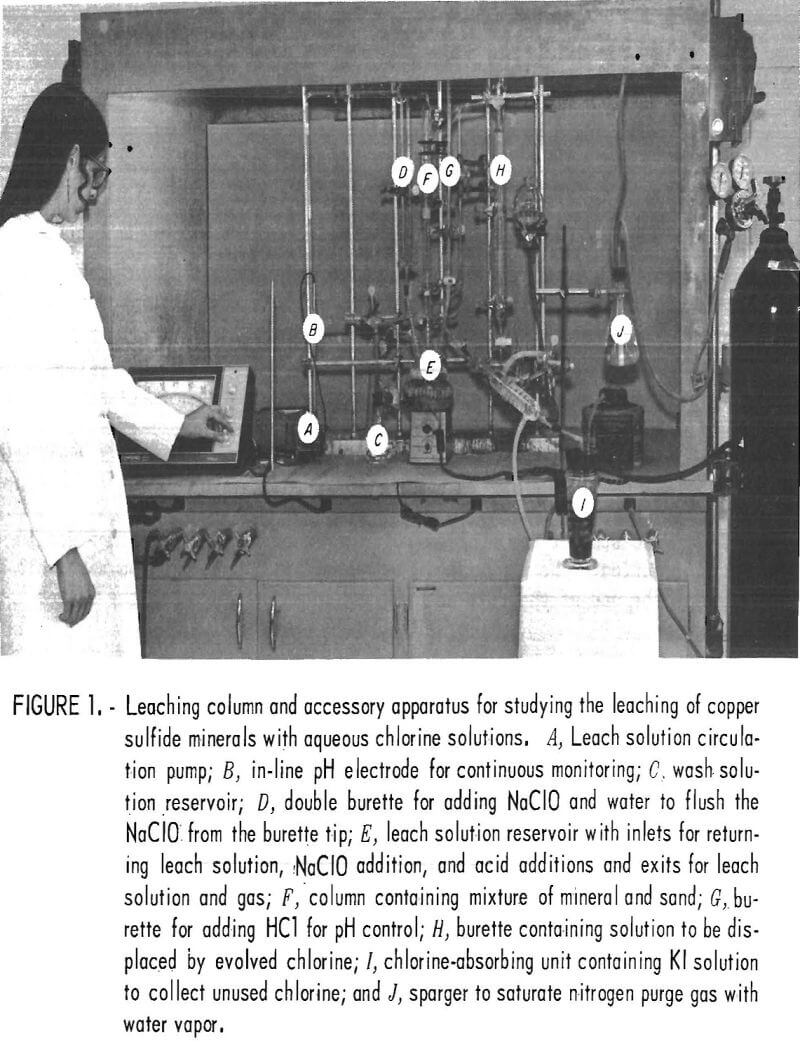
Figure 1 shows the column leaching apparatus and the accessory equipment used for the controlled leaching experiments.
A magnetic stirrer was used to aid in redissolving the chlorine gas in the leach solution. The heating mantle around the leach solution reservoir was used at the end of the test to heat the solution and expel the unused chlorine.
A downward percolation method of leaching was selected for this study. The sulfide minerals were mixed with 20-mesh precleaned sand and leached at ambient temperature and pressure by circulating the acid-chlorine solution through the mixture bedded in a glass column (designated F in fig. 1). Dispersing the mineral particles in a sand matrix insured uniform contact with the leach liquor and avoided particle abrasion which is inherent to an agitated leaching system. However, tests were not made to compare the merits of various leaching techniques.
To initiate a leaching test, a mixture of 70 grams of sand and 1 gram of sulfide mineral was charged to the leaching column, 60 ml of distilled water was added to the leach solution reservoir, the apparatus was assembled, and the system was purged with nitrogen for 30 minutes. The elimination of oxygen from the system by the nitrogen purge was an arbitrary condition, and the effect of oxygen was not determined. After purging and closing the system, measured volumes of NaClO and HCl were introduced to the solution reservoir to produce the desired chlorine solution. The sulfide minerals were leached by circulating the acid-chlorine solution through the column of sand-mineral mixture. A leaching stage was 1 hour, and for multiple-stage tests, NaClO additions were made without opening the system and 1-hour leaches were repeated. After leaching, solution circulation was discontinued and the system again purged with nitrogen to expel unused chlorine, which was collected by the potassium iodide solution in the gas absorbing unit. During this purge, the entire system was washed and the leach solution boiled to drive off excess chlorine. Leach solutions were analyzed for copper, iron, and sulfate ions. Potassium iodide solution from the gas absorber was titrated with standardized thiosulfate solution to determine the amount of unused chlorine. The chlorine used was determined by the difference between the chlorine content of the sodium hypochlorite put into the system and the unused chlorine recovered in the potassium iodide solution.
The validity of the test procedure was determined by conducting a blank test in which the sulfide mineral was omitted. The results of this test showed that more than 99 percent of the chlorine input was recovered in the gas absorber thus demonstrating the accuracy of the procedure.
Experimental Results
The leaching solution pH for the initial tests was adjusted to 2.0 and, as the leach progressed, acid formation caused the pH to decrease to about 1.0. In this pH range, the predominant oxidizing species was dissolved chlorine. Assuming that chlorine was the oxidant and that the reaction products (copper, iron, and sulfur) were in their highest oxidation states, postulated chemical equations representing leaching reactions for the various sulfide minerals are as follows:
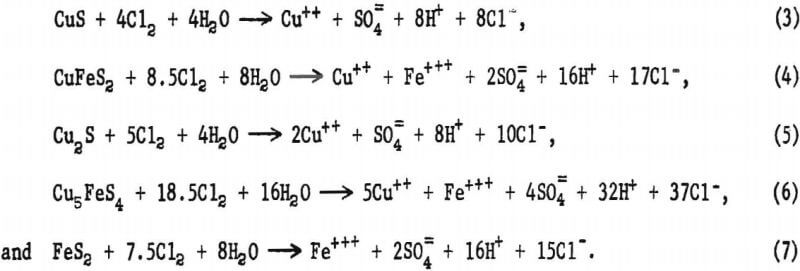
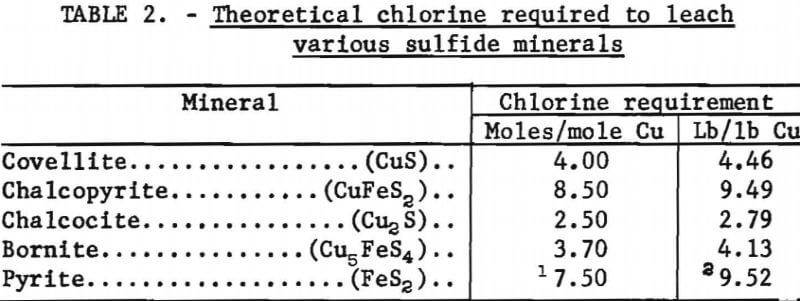
The theoretical amount of chlorine required to leach each of the sulfide minerals is given in table 2.
Leaching Copper Sulfide Minerals
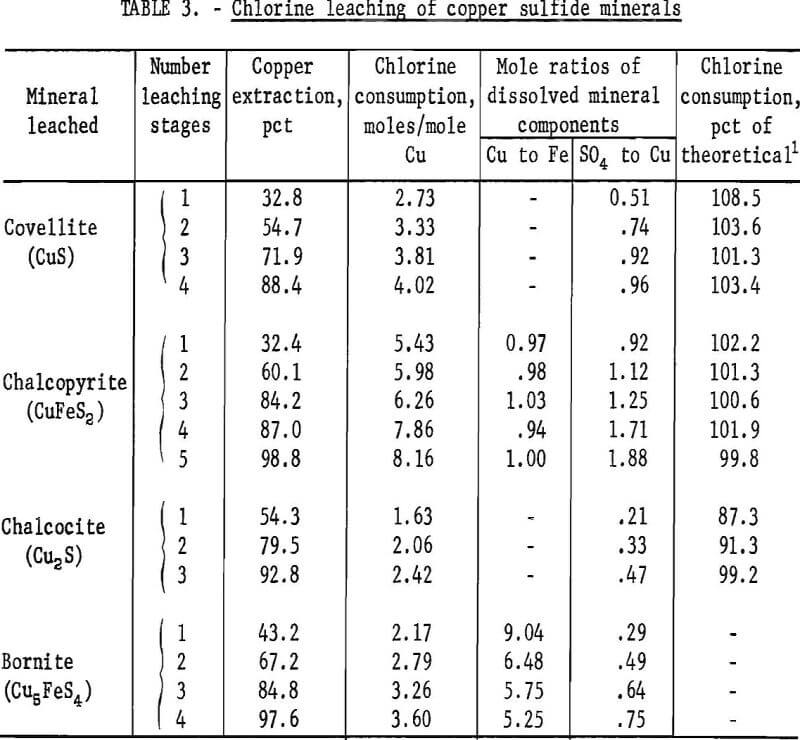
Each of the copper sulfide minerals was leached by stages in the column reactor with aqueous chlorine solutions. The sample contained 1 gram of mineral and each leaching stage consumed about 0.01 mole of chlorine. Enough leaching stages were used to attain near complete copper extraction with each mineral. Results of these tests are presented in table 3.
Discussion of Results
Covellite
The covellite leaching test results showed that with one stage of leaching 32.8 percent of the copper was extracted, and the extraction increased to 88.4 percent as the number of stages was increased to four. Also, the chlorine consumption increased from 2.73 to 4.02 moles per mole of copper, as the number of leaching stages was increased. Thus, by extrapolation, a leaching system that dissolves all of the copper will use about 4 moles of chlorine to extract 1 mole of copper, or about 4.46 pounds of chlorine per pound of copper.
The low ratio of chlorine-to-copper obtained for the early leaching stages was probably due to incomplete oxidation of the sulfur species. Equation 3 shows that the sulfur is oxidized to sulfate, and the mole ratio of sulfate-to-copper should be 1.0. The test data showed mole ratios of 0.51, 0.74, 0.92, and 0.96, respectively, for one through four leaching stages.
Thus, with incomplete leaching, there was less sulfate in the system than was predictable from equation 3 and, as leaching approached completeness, the stoichiometric ratio of sulfate-to-copper approached the theoretical value of 1.0. In the early stages of leaching, considerable sulfur must have been in a state intermediate between sulfide (S=) and sulfate (S+6).
Sulfur from these partly oxidized sulfides probably was in the elemental form, and an examination of a ½-inch piece of covellite after leaching revealed that the mineral surface was coated with elemental sulfur.
Calculations were made to determine if the chlorine consumption could be correlated to the formation of elemental sulfur and sulfate as the only oxidation products. The amount of elemental sulfur present was determined by obtaining the difference between the actual mole ratio of sulfate to copper and the theoretical ratio of 1.0. The following stoichiometric relationships were used to calculate the theoretical chlorine consumed by each sulfur product:
S= + Cl2 → S° + 2Cl-…………………………………………………..(8)
and S= + 4Cl2 → S+6 (sulfate) + 8Cl-…………………………..(9)
The actual chlorine consumptions were compared to these calculated values and the results are included in table 3 under the heading “Chlorine consumption.” The data reveal that the actual chlorine consumption varied from 101 to 108 percent of the theoretical chlorine consumption that could be accounted for by these two sulfur products. The closeness of these values to 100 percent indicates that the predominant intermediate sulfur species is elemental sulfur. The greater-than-100-percent value of chlorine consumption generally found may mean that a small proportion of the sulfur was in an oxidation state between the elemental and sulfate form; however, products of this type were not identified. These products, if present, were transitory, their amount small, and would probably be of only minor concern in a leaching process.
Chalcopyrite
The data from the chalcopyrite leaching tests showed trends similar to the covellite leaching data. As copper extraction increased from 32.4 to 98.8 percent, the chlorine consumption increased from 5.43 to 8.16 moles per mole of copper leached. The previously determined theoretical requirement was 8.50 moles of chlorine per mole of copper which was the value approached as the copper leaching neared completion.
From equation 4, the mole ratios of copper-to-iron and sulfate-to-copper in the leach solution should be 1.0 and 2.0, respectively. The data in table 3 show that, within the limits of estimated experimental error, the copper-to-iron ratio was 1.00 and did not vary as the amount of the mineral leached increased. Therefore, chalcopyrite was leached in stoichiometric proportions. The sulfate-to-copper ratios varied from 0.92 to 1.88, and these less-than-theoretical values indicate that the oxidation of sulfur to sulfate was incomplete. The intermediate form for the sulfur was assumed to be elemental sulfur as with the covellite, and this assumption was substantiated by microscopic examination of leached chalcopyrite particles.
Further calculations were made, comparable to those made with the covellite leaching data, to determine whether the actual chlorine used could be correlated to the formation of elemental sulfur and sulfate as the only sulfur product. These data show that 99.8 to 102.2 percent of the chlorine used was accounted for. This was good correlation and, when considered in conjunction with the other evidence, indicates that elemental sulfur was the predominant intermediate form of sulfur.
Chalcocite
The data from the chalcocite experiments showed that as copper recovery increased from 54.3 to 92.8 percent, the chlorine requirement increased from 1.63 to 2.42 moles per mole of copper. As with the other minerals, the chlorine consumption approached the theoretical value (2.50 moles per mole of copper) as copper extraction approached 100 percent. The mole ratios of sulfate-to-copper in the leach solution showed that all of the sulfur had not been oxidized to sulfate, but when the assumption was made that the only intermediate form was elemental sulfur, the actual chlorine used could not be satisfactorily accounted for. The amount of chlorine theoretically needed to form elemental sulfur was more than the amount used, which meant that some of the sulfur in partly reacted sulfides had not been oxidized to the elemental form. Conceivably, the chalcocite was leaching and altering to covellite and then the covellite was leaching with the formation of elemental sulfur. The following chemical equations represent these reactions:
Cu2S + Cl2 → Cu++ + 2Cl- + CuS………………………………………………..(10)
CuS + Cl2 → Cu++ + S° + 2Cl-…………………………………………………….(11)
and S° + 3Cl2 + 4H2O → SO4= + 6Cl- + 8H+………………………………(12)
Microscopic examination of a leached chalcocite specimen revealed that the mineral surface was coated with elemental sulfur. A polished, cross section of the leached mineral showed definite alterations from the surface inward, and two alteration products, in addition to elemental sulfur, were noted. The product adjacent to the leached surface was identified as covellite (CuS) and the other alteration product between the covellite and chalcocite mineral appeared to be digenite (Cu1.8S).
The mechanism by which chalcocite leached in an aqueous chlorine system was complicated, but the experimental data showed that the overall reaction was fairly represented by equation 5.
Bornite
The chlorine requirement for leaching bornite also approached the theoretical value (3.70 moles per mole copper) as 100 percent copper extraction was approached. With one stage of leaching, 43.2 percent copper extraction was attained and the chlorine consumption was 2.17 moles per mole copper, and with four leaching stages, the copper extraction was 97.6 percent, and 3.60 moles of chlorine were required for each mole of copper leached.
The mole ratios of copper to iron leached were more revealing as to the complex nature of the bornite leaching reactions. From the molecular formula of bornite (Cu5FeS4), the mole ratio of copper-to-iron should be 5.00, but with one stage of leaching, the ratio was 9.04. Almost twice as much as the predicted amount of copper had leached, or stated another way, only one-half as much iron as predicted had leached. Thus, the amount of sulfur leached from the mineral could not be predicted, the distribution of the leached sulfur between the elemental form and sulfate could not be determined, and a basis for calculating the percent of theoretical chlorine utilization for partly leached bornite could not be established.
Microscopic examination of a piece of leached bornite revealed a surface coating of elemental sulfur, and a polished cross section showed a zone of altered material from the surface inward. The composition or identity of the altered material was not determined, but it was probably a form of nonstoichiometric copper-deficient bornite.
Leaching Pyrite
Pyrite is commonly associated with copper sulfide minerals and a study of copper leaching would not be complete without including the leaching deportment of this iron sulfide mineral. Therefore, high grade samples of pyrite were leached with aqueous chlorine solutions by the same procedure and test conditions as used with the copper minerals. Six stages of leaching were required to attain the desired high-iron extraction. The results are tabulated in table 4.
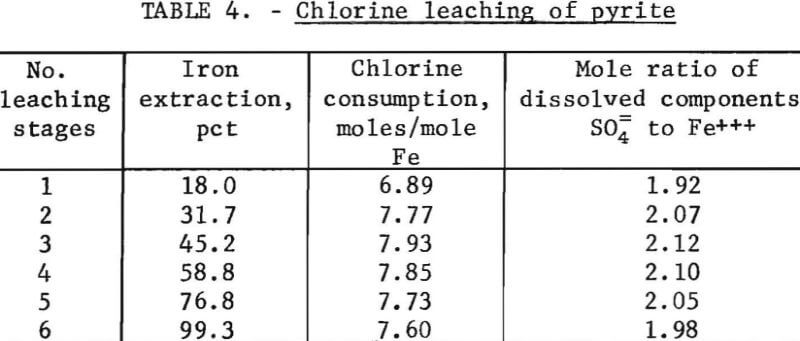
The reaction of chlorine with pyrite is given by equation 7 and requires stoichiometric ratios of chlorine-to-iron and sulfate-to-iron of 7.5 and 2.0, respectively. The experimental data did not deviate greatly from these values for the various number of stages leached. Thus, it appeared that pyrite leached in stoichiometric proportions and that the only sulfur species formed was sulfate. However, microscopic examination of a leached polished surface of a large piece of pyrite revealed the presence of considerable elemental sulfur. Elemental sulfur on the pyrite surface indicated that the simple reaction deduced from the leaching data was incorrect. Apparently, the pyrite altered to FeS and S°, and these two components were leached at approximately the same rates. The equations representing this process are as follows:
FeS2 → FeS + S°…………………………………………………………………………(13)
FeS + 4.5 Cl2 + 4H2O → Fe+++ + SO4= + 9Cl- + 8H+………………….(14)
and S° + 3Cl2 + 4H2O → SO4= + 6Cl- + 8H+……………………………….(15)
Effect of pH Variations
Single stage leaching tests were made with each of the minerals to determine the effect of variations in leach solution pH. The pH values of the solutions used were 0.50 to 0.25 and 4.0 to 2.0. The results corresponded with the previously reported results obtained with solution pH of 2.0 to 1.0; pH values greater than 4.0 were not included in this study because precipitation of iron would interfere with sampling and analysis of the test data.
The test results over the wider pH span were about the same as at pH 2.0 to 1.0. Apparently, close pH control within the range pH 0.25 to pH 4.0 is not necessary for leaching copper and iron sulfide minerals with aqueous chlorine solutions.
Conclusions
The investigation has shown that copper sulfide minerals and pyrite are readily oxidized and dissolved by acidic chlorine solutions at ambient temperatures. With complete mineral dissolution, the reaction products were in their highest oxidation state and the stoichiometric amount of chlorine was consumed.
Covellite and chalcopyrite leached in a relatively simple manner with only elemental sulfur as an intermediate product. However, the elemental sulfur was oxidized to sulfate as the leach progressed to completion. Copper extraction from covellite and chalcopyrite required 4.46 and 9.49 pounds of chlorine per pound of copper, respectively.
Chalcocite and bornite leached in a complicated manner. In addition to forming elemental sulfur as an intermediate product, the chalcocite was altered to covellite and another solid product which most probably was digenite. The solid phase alteration product from bornite was not identified but appeared to be a nonstoichiometric copper-deficient form of the mineral. The chlorine requirements for leaching chalcocite and bornite were 2.79 and 4.13 pounds per pound of copper extracted, respectively.
Pyrite appeared to alter initially to FeS and S°, and these two components leached at approximately the same rates. Pyrite required 9.52 pounds of chlorine per pound of iron leached. This would be a costly consumption of chlorine in a copper leaching process using high-pyrite ores or concentrates.
