Table of Contents
Two of the missions of the Bureau of Mines are to conserve scarce, costly metals and to reduce pollution. In accordance with these missions, the objective of this investigation was to develop a method of recycling spent chromium etching solutions that are now discarded as wastes.
Solutions containing hexavalent chromium (as chromic acid or sodium dichromate) and sulfuric acid are used in brass finishing, printed circuit board etching, preparation of plastic for plating, anodizing, and various other surface treatments. As the solutions are used Cr6+ is reduced to Cr3+, the dissolved solids content increases, and the effective acid concentration is reduced. The solutions are discarded when the etching rate is below the rate required for a given operation. Technology for ultimate disposal of chromium wastes usually involves the reduction of the remaining Cr6+ to Cr3+, followed by base addition to precipitate chromium and other metallic hydroxides. The resulting sludge is discarded in landfill sites.
The use and disposal of chromic acid-sulfuric acid etchants in this manner presents significant problems in secondary resource recovery and pollution control. All U.S. primary chromium production is from imported ores. More than 2.7 x 10 7 kg of chromium is used annually for metal surface treatment and corrosion control measures. The minimal recovery technology and practices of the metal-finishing industry result in the discarding of valuable secondary resources such as chromium. In addition, chromium has long been recognized as a major pollution problem. Current Environmental Protection Agency (EPA) regulations prohibit sewage disposal of waste water containing more than 0.25 mg/l Cr6+ by plants discharging more than 152,000 l/day. Landfill areas suitable for chromium hydroxide sludges are becoming scarce, and collection, treatment, and disposal by waste contractors is expensive.
For these reasons, it is desirable to develop a method for economical, in-plant recycling of spent chromic acid-sulfuric acid etchants. The spent etchants discussed here are typical of those used in the brass and printed circuit board industry. Rinse waters from plastic-etching operations were also examined. Recycling such etchants would result in a major reduction of chromium-containing effluents, thus reducing costs of waste treatment and disposal. Further, recycling these etchants would conserve chromium, thus reducing process costs by reducing chromium purchases.
The requirements for an in-plant recycling process are in some respects more stringent than those for a simple metal recovery process. Reagent additions are limited because such additions would necessitate reagent removal.
Any volume increase or chromium concentration decrease would require some type of evaporative recovery system. An electrochemical approach was chosen to circumvent these difficulties. Etchants used to prepare plastic for plating present a different problem: Their high Cr6+ concentrations can corrode equipment used to regenerate the etchants.
Since many plastic etching operations now use countercurrent rinsing coupled with evaporative recovery to recycle the etchant, it was suggested that electrochemical technology be used to regenerate the rinse. The rinse would then be concentrated and returned to the etching tank.
Several electrolysis methods have been proposed for recycling etching solutions. Lancy used an electrodialysis cell fitted with a cation- selective membrane to separate copper from chromic acid. The copper was recovered by electrowinning in a separate unit. Tirrell suggested reduction of the Cr6+ content of a spent etchant to Cr3+ in the cathode compartment of a diaphragm cell. When all the Cr6+ was reduced, the copper was plated out at the cathode. The copper-deficient solution was then used as the anolyte, and the Cr3+ was oxidized back to Cr6+. Fujii suggested a similar approach for exhausted chromium-plating solutions. But none of these approaches make optimum use of the required electrical energy.
The process described herein differs from previous processes in that the chromium is oxidized and contaminating metals are removed in a one-step process that minimizes the use of electrical energy. The process employs a diaphragm cell fitted with a cation-selective membrane. Spent etchants and rinse waters from plastic plating operations are placed into the anode compartment where Cr3+ is oxidized to Cr6+. The effective acid concentration increased to the normal value for a comparable fresh solution (120-300 g/l H2SO4). A portion of the copper (and zinc in a brass etchant) is transferred to the catholyte. Essentially all the copper is recovered on the cathode. (No zinc plates out under these conditions.)
The Regeneration Experiment
Spent Etchants
The composition of several spent etchants are given in table 1. The designations A through D refer to different firms. Samples A and B are spent brass etchants. Sample C is a spent printed circuit board etchant. These solutions were originally prepared from sodium dichromate and sulfuric acid. Sample D is not an etchant but is obtained from the first countercurrent rinse tank following a plastic-etching operation. The etchant originally was prepared from chromic and sulfuric acid and contained about 280 g/l Cr6+ and 430 g/l sulfuric acid.
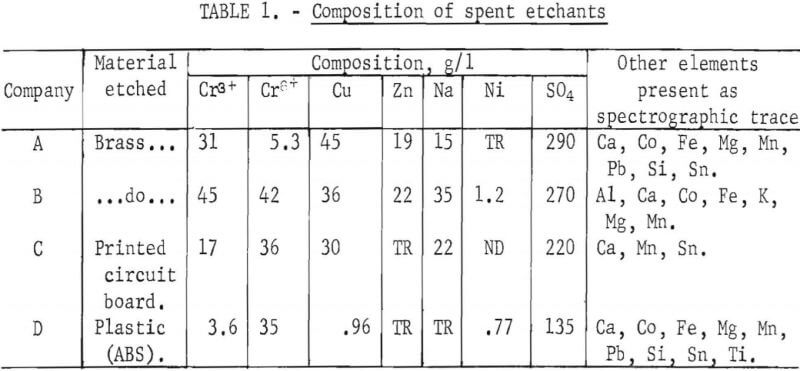
The Diaphragm Cell
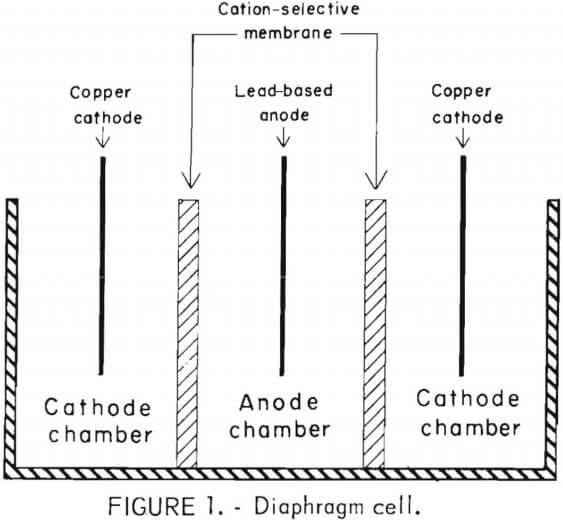
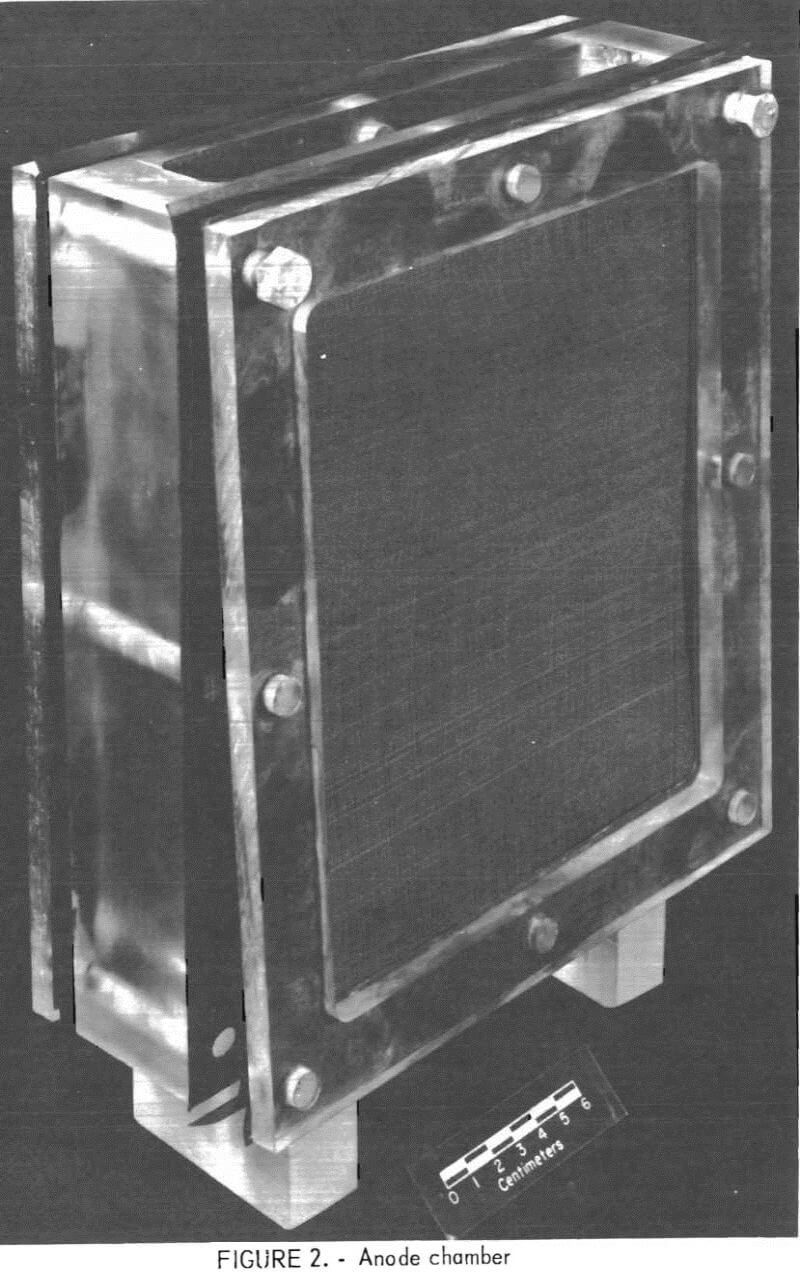
The general arrangement of the diaphragm cells used in this research is shown in figure 1. The anode chamber is fitted with cation-selective membranes to allow ion migration without mixing the anolyte (the spent etchant) and catholyte (180 g/l sulfuric acid). The chamber is shown in figure 2. Lead-based anodes and copper cathodes were used. The areas of the anode, cathode, and membrane are equal. Cathode current density is 2.15 amp/dm². The cell may be operated batchwise or continuously. Continuous operation of the diaphragm cell is illustrated in figure 3. All etchants were filtered through a 1-µm-pore-size polypropylene cartridge before treatment. This reduced membrane fouling and lowered cell resistance substantially.
Results and Discussion
Bench-Scale Studies
Oxidation of Cr3+ to Cr6+ was accomplished for all samples of spent etchant without difficulty. One hundred percent of the Cr3 that remained in the anolyte was oxidized in all samples. The initial 80 pct of the Cr3+ in any of the spent etchants was oxidized at 90-pct anode efficiency. The oxidation is relatively independent of membrane type, concentration of sulfuric acid in the catholyte, and current density. The rate of Cr3+ oxidation is much faster than the rate of zinc and/or copper ion transfer through the membrane. Table 2 gives the composition of etchants A through D after regeneration. These are the results of static tests in which 0.87 1 of etchant was treated for 24 hours (samples A through C). Sample D was treated for only 6 hours because it was much more dilute. Ionics type-61 cation-selective membranes were used in the treatment of samples B and C. Samples A and D were treated using Nafion membranes. The higher chromium loss probably is the result of leaks around the membranes. Nafion is thinner than the Ionics membrane, which makes it more difficult to achieve a good seal. But Nafion was preferred because of its high oxidation resistance.
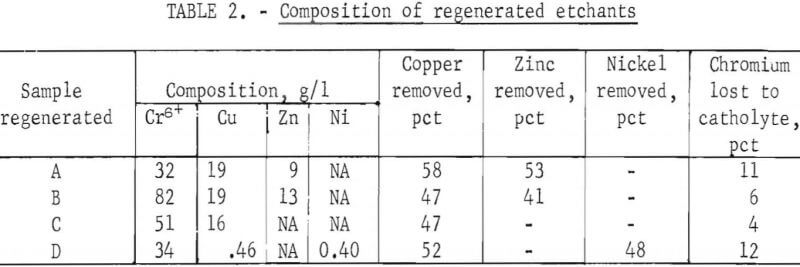
Cathode current efficiency ranged from 30 pct initially to 5 pct when one-half of the copper had been removed. Although cathode efficiency was low, the cost of oxidizing the Cr3+ is estimated at only 6.6 kwhr/kg of sodium dichromate regenerated for samples A, B, and C. About 8.8 kwhr/kg of sodium dichromate regenerated was required for sample D. The cost estimate is based on the cell voltage (about 3 v) and current (14 amp). As the membrane material becomes conditioned and the concentration of copper and zinc in the cathode chamber increases, the voltage decreases and rapidly reaches a constant value. This constant value is maintained during the final 90 pct of the static tests.
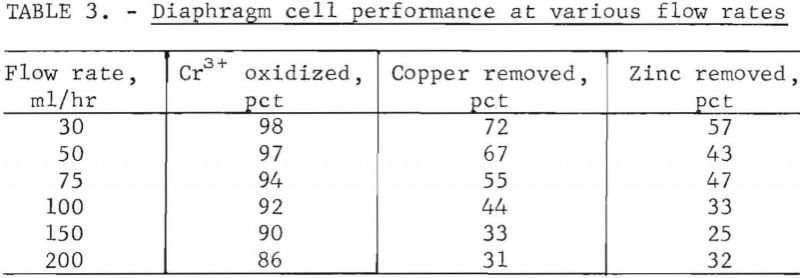
Table 3 shows the performance of the diaphragm cell at various etchant flow rates for sample A. As the flow rate was increased, the percent of the chromium that was oxidized decreased relatively slightly. This implies that the cost of regeneration of hexavalent chromium decreases as the requirements for removal of contaminating metals decrease, because of the shorter retention time required.
Samples of spent brass etchant regenerated at the Rolla Metallurgy Research Center have been evaluated by two brass-producing companies. Both reported that the regenerated etchant, when diluted to the proper operating level, etched brass as well as a fresh solution. In addition, the regenerated etchant was found to be superior to a fresh etchant in removing “red stain” (a product of oxidation) from brass. A dilution is necessary because of the method used by the brass-producing companies in operating their etching tanks. As the workpieces move through the tank, the etchant becomes spent (1) by drag-out and water additions to bring the solution level up to operating level, and (2) through the reduction of Cr6+ to Cr3+ by the chemical action of the etchant. The operator must add sodium dichromate and sulfuric acid at intervals to make up these losses. As the tank is operated, the composition of the etchant changes and the specific gravity of the solution increases. When the etching operation can no longer be made satisfactory with chemical additions, the tank is emptied.
Two modes of operation are possible with brass and printed circuit board etchants using the Bureau-developed etchant regeneration technique:
1. The etchant may be used until completely spent and then regenerated. After regeneration, the solution can be diluted to the proper operating level. Chemical consumption and dragout can be compensated for by additions of the concentrated etchant.
2. The regeneration unit may operate continuously in direct connection with the etching tanks. Drag-out 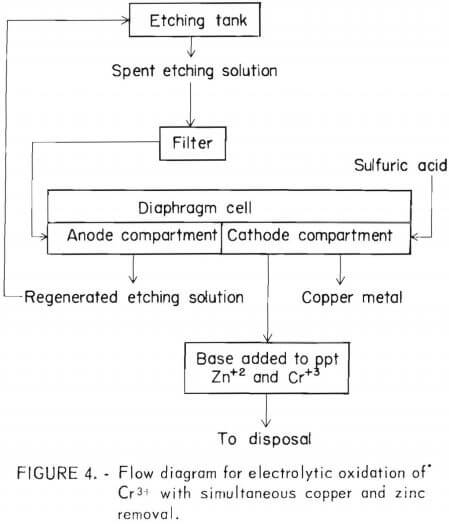 would still have to be counteracted by chemical additions however, the chemical content of the solution would reach a constant value, and the specific gravity of the solution would be lowered. This would minimize dragout. Figure 4 shows a flowsheet compatible with either mode of operation.
would still have to be counteracted by chemical additions however, the chemical content of the solution would reach a constant value, and the specific gravity of the solution would be lowered. This would minimize dragout. Figure 4 shows a flowsheet compatible with either mode of operation.
In the case of regenerating rinse waters in plastic etching operations, the unit would operate continuously in direct connection with the countercurrent rinsing system. After the rinse was regenerated, it would be concentrated by evaporative recovery. Many plastic-etching operations are already equipped with countercurrent rinsing and evaporative recovery systems.
The advantage of the regeneration unit is that no Cr3+ would be returned to the process tank and substantial amounts of copper and nickel (dissolved from the plating racks) would be removed.
The regeneration system produces a catholyte bleed stream that contains a small amount of Cr3+ and other metals that do not plate under these conditions. This is a small loss of metal compared with dumping the entire spent etchant. The purpose of the bleed stream is to maintain good contaminant metal removal from the spent etchant. It is replaced with a sulfuric acid solution. A continuously generated stream of this composition would put far less strain on a plant’s waste treatment facilities than the regular dumping of a large amount of concentrate spent etchant.
Large-Scale Studies
A process research unit was designed and constructed to demonstrate the viability of the regeneration technique on an industrial scale. The diaphragm
cell in this system is 0.91 by 0.91 by 0.91 m, contains two anode chambers, and requires approximately 500 amp at approximately 3 volts. With the exception of the multiple anode chambers, construction of the diaphragm cell in the process research unit is similar to that used in the bench-scale studies.
Figure 5 is a view of the process research unit showing (from left to right) an in-tank filter unit, etchant holding tank, control panel, and catholyte circulation tank. An overhead view of the diaphragm cell is shown in figure 6. The tank to the right of the electrodes serves to minimize the volume of catholyte needed. The space that the small tank occupies can hold three more anode chambers, thus expanding the research unit to 2.5 times its present capacity. Figure 7 shows one of the cathodes being positioned in the cell.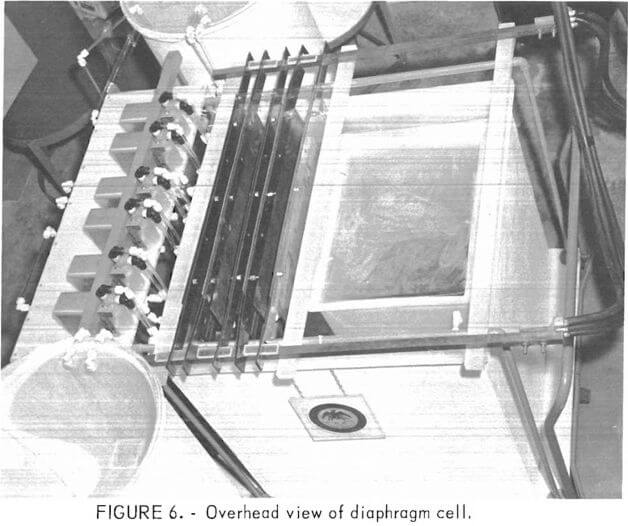
In a preliminary test, the research unit was operated continuously for 72 hours using a spent brass etchant containing, in grams per liter: 23 Cr3+, 8.2 Cr6+, 28 Cu2+, and 13 Zn2+ Cr3+ oxidation averaged 98 pct. Copper and zinc removals were both 41 pct. Energy consumption was 8.6 kwhr/kg sodium dichromate produced. Copper powder, containing 90 pct copper, was recovered from the cathodes, Carbon (2 pct) was added and the material was melted without agglomeration in an induction furnace to produce an ingot containing 99.7 pct copper.
Summary
The technical feasibility of regenerating and recycling spent chromic acid-sulfuric acid etchants has been demonstrated. Using a diaphragm cell, 100 pct of the Cr3+ was oxidized to Cr6+ while substantial amounts of copper, zinc, and nickel were removed. Results using regenerated etchants were excellent, according to industry evaluations. A unit capable of regenerating spent etchants on an industrial scale has been constructed and operated.
Etchants containing hexavalent chromium and sulfuric acid are used in a variety of surface-finishing operations. When the resultant spent solutions are discarded, substantial quantities of chromium are lost and pollution problems are created. To minimize the undesirable environmental effects while improving metal and minerals recycling technology, the Bureau of Mines is conducting research on a method for the economic implant recycling of these waste etchants. Trivalent chromium, produced when the etchant reacts with a substrate, is oxidized in the anode chamber of a diaphragm cell; other metals, dissolved during the etching operation, are transferred to the catholyte.
When waste brass etchants are treated in this manner, all of the trivalent chromium is oxidized and more than 40 percent of the copper and zinc is removed. Similar copper removal and chromium oxidation are achieved with waste printed circuit board etchants and rinse waters from plastic etching operations. The electrical energy needed to regenerate 1 kg sodium dichromate is less than 9 kwhr. Regenerated brass etchants evaluated by two companies equaled or exceeded the performance of fresh etchants.
