Table of Contents
The dissolution behavior of silver and copper from silver/copper alloys in ammoniacal solutions has been investigated using a rotating disc. Oxygen was used as an oxidant. The effect of composition of alloys, concentration of lixiviants, stirring speed and temperature on the overall dissolution kinetics has been studied.
Theoretical Background
The dissolution of copper and silver in ammonia solution can be described as follows:
Ag + 2NH3 + ¼O2 + ½H2O = Ag(NH3)2+ + OH-…………………………………………………….(1)
Cu + 4NH3 + ½O2 + H2O = Cu(NH3)4²+ + 2OH-……………………………………………………(2)
There are two kinds of alloys, namely homogeneous and heterogeneous alloys. Homogeneous alloys are represented by solid solution comprising of only one phase, while heterogeneous alloys are mixtures of two or more separate phases. Solid-solution alloys are usually more corrosion resistant or more difficult to leach than alloys with two or more phases, since galvanic coupling effect is not present.
The dissolution of metals in aqueous solutions can be broken down into five steps:
- Diffusion of the reactant(s) from bulk of the solution to the metal surface through the boundary layer.
- Adsorption of the reactant(s) onto the metal surface.
- Chemical reaction at the surface.
- Desorption of the soluble product(s).
- Diffusion of the product(s) through the boundary layer back to the bulk solution.
Experimental
A known composition of silver and copper powders was mixed and heated to about 1500° C. The molten liquid mixture was poured into a cylindrical mold followed by air quenching.
Figure 1 shows the schematic representation of the experimental set up.
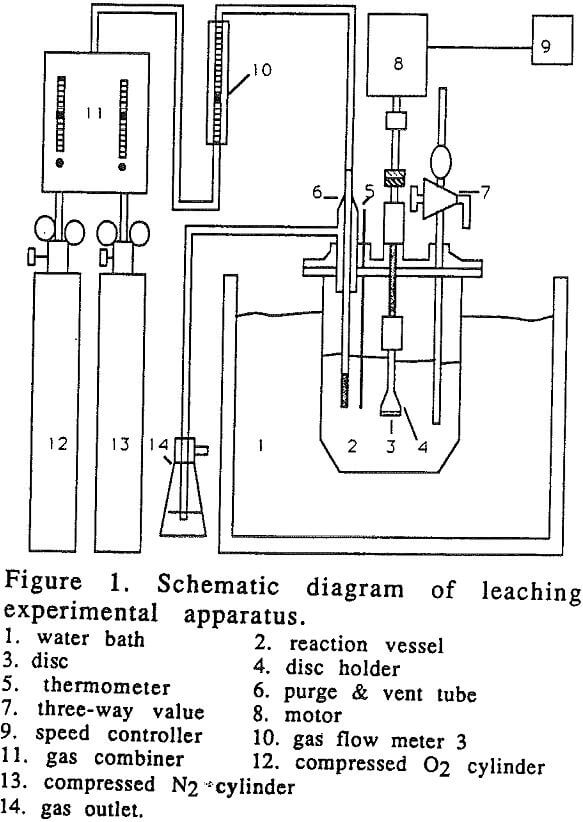
Leaching experiments were carried out in a one liter glass vessel. Leaching solutions were prepared by dissolving known amounts of reagent-grade ammonium hydroxide in distilled water, which was purged for one and a half hours with air or a mixture of oxygen and nitrogen with an appropriate oxygen concentration.
Results and Discussion
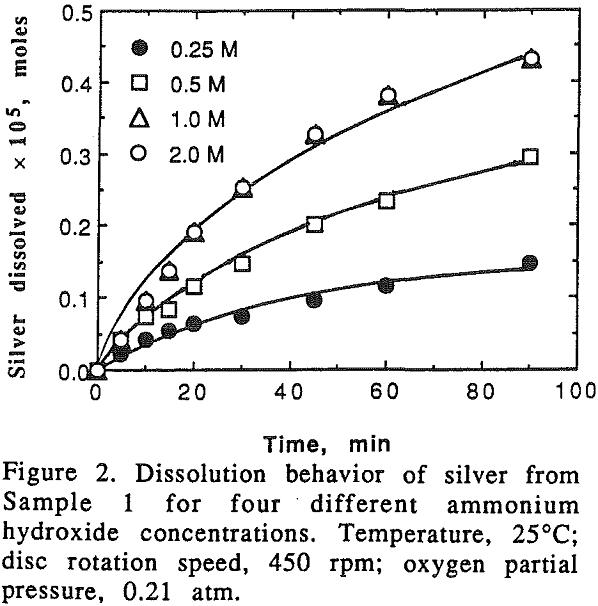
A series of experiments were carried out in which the oxygen pressure was maintained at 0.21 atm, and the ammonium hydroxide concentration was varied from 0.25 M to 2 M. The dissolution rate of silver fell off with time for pure silver, while the rate increased with time when it was leached from silver-copper alloys. On the other hand, the dissolution rate of copper was linear with time for pure copper, and fell off with time for the alloys.
When pure silver was dissolved, the color of the disc was changed to light yellow after the leaching. This was attributed to the fact that a passive film was formed during the dissolution process under the experimental conditions. Such a passive film undoubtely slowed down the dissolution rate.
Silver-copper alloys are mixtures of two separated phases (Ag and Cu). A potential difference existed between the two dissimilar phases when the alloy was immersed in the leaching solution. The more noble Ag-phase became cathodic and the less noble Cu-phase became anodic.
It can be seen that the dissolution rate of silver from pure silver and alloys increases with the increase of the ammonium hydroxide concentration and the slope of the curves decreases with the decrease of the silver content in the alloy. It should be noted that when silver content in the alloy was 24.1%.
The dissolution rate of silver from pure silver and silver/copper alloys were chemical reaction controlled. The dissolution rate of copper from pure copper and silver/copper alloys were oxygen diffusion controlled.

