The increasing demand for greater speed and more accuracy, in making daily assays of ores and products from mills treating material containing but very small quantities of lead and copper, has caused the older analytical methods to be supplanted by new ones devised to meet the needs of modern work.
For “ control ” work in copper-analyses, I had, previous to the present equipment, introduced in my laboratory the ordinary electrolytic method, using hollow cylindrical electrodes; and with these I began a series of experiments in determining the lead-content of daily mill-samples and controls. The results, proving accurate and satisfactory, I then devised, with Mr. H. E. T. Haultain, a cheaper electrode than the ordinary jacket-electrode, which costs more and more,—the expense of the older form making the installation of many units very costly.
The form of cathode finally adopted as the most satisfactory, shown in Fig. 1, is cut from 0.001-in. platinum foil. It is 12.5 cm. long, and is divided into a blade 4 cm. wide and 6.25 cm. long, and a tongue 0.7 cm. wide and 6.25 long, the immersion area being 50 sq. cm. and weight 1.5 grams. The blade is first sand-blasted and then corrugated lengthwise, in order to impart the necessary rigidity. Strips of platinum foil 0.001 in. thick, 12.5 cm. long and 0.5 cm. wide, having a median corrugation, form the anodes. Three electrodes are used in each cell; one anode in the middle and one cathode on each side of the anode. These electrodes are connected to slotted aluminum terminals, in which they are held by contact pressure. The terminals are 5/16-in. rods, projecting 2 in. horizontally in front of the wall of the cabinet; at the back the middle electrode (anode) is connected with one pole of the current, and the two outer ones (cathodes) with the other pole.
The present equipment in the laboratory consists of two cabinets of 30 cells each, one used for lead, and the other for copper-determinations. Each cabinet is individually wired in series, but both are connected in multiple and receive a current of 130 volts, which is controlled by enameled field-rheostats, one for each cabinet. (See Fig. 2.) Formerly, lamps were used to furnish the resistance. One voltmeter and one ammeter reading to 0.02 amperes is sufficient for both cabinets.
Exclusive of wiring at and from the power-house, the cost of installation, made by Mr. Haultain and myself, was much less than $200. The platinum, ordered in sheets, was cut, sand-blasted and corrugated. The aluminum was purchased in ingots and cast into terminals. A carpenter took three days to make the cabinets. With this equipment I make an average of 45 lead- and 50 copper-determinations daily, besides attending to other work of the office.
I am not aware that any other electrolytic method is in practical daily use for the estimation of lead. In devising my method I have referred to the published articles of various chemists, and to the books of Classen, Neumann and Smith bearing on the subject. Briefly, the method is as follows:
The ore, weighed into a tall battery-beaker of 100-cc. capacity, is digested with 10 cc. of nitric acid. The lead sulphate formed is readily dissolved after boiling by the addition to the beaker of from 10 to 20 cc. of a saturated solution of ammonium nitrate containing 20 per cent, of free ammonia. After solution the beaker is nearly filled with water, and from 10 to 20 cc. of nitric acid added. The solution is now ready for electrolysis. A wide range of current-strength is permissible, but from 1.5 to 2 amperes is most satisfactory; this amount keeps the solution sufficiently hot during the electrolysis. At the end of two hours, the lead is completely deposited in the form of peroxide on the anode. The anode is then removed, washed in water and in alcohol, ignited and weighed. The theoretical factor is 0.866; but in practice 0.855 is found to be more accurate; probably due, as Hollard says, to an excess of oxygen in the peroxide.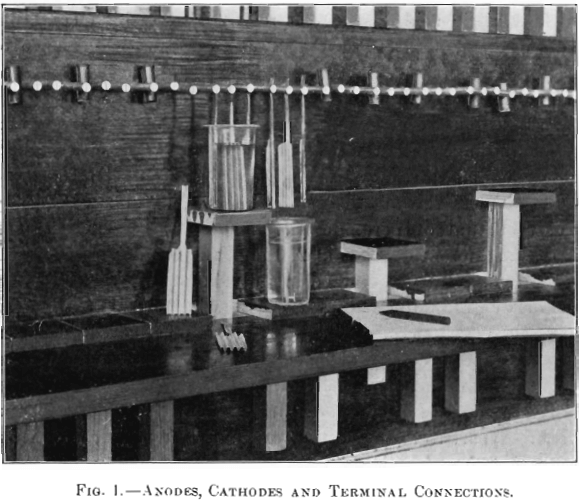
The accuracy of this method ranks with that of carefully- made electrolytic coppers, and its great advantage is the small amount of the chemist’s time required. In the presence of manganese or antimony, it is necessary to have a large excess of free nitric acid in the electrolyte, under which condition neither element interferes. Bismuth, even in the presence of very large amounts of free nitric acid, is partly precipitated as bismuth oxide with the lead. Its presence can be recognized by a light-blue color given to the peroxide coat. Arsenic and tellurium have to be removed before electrolysis, for, if present in large amounts, they effectually prevent any deposition of lead. Unless the anode is sandblasted, only a comparatively small amount of peroxide will adhere, but, properly sandblasted, adherent deposits of 250 mg., and even up to 600 mg., of peroxide may be obtained in daily work.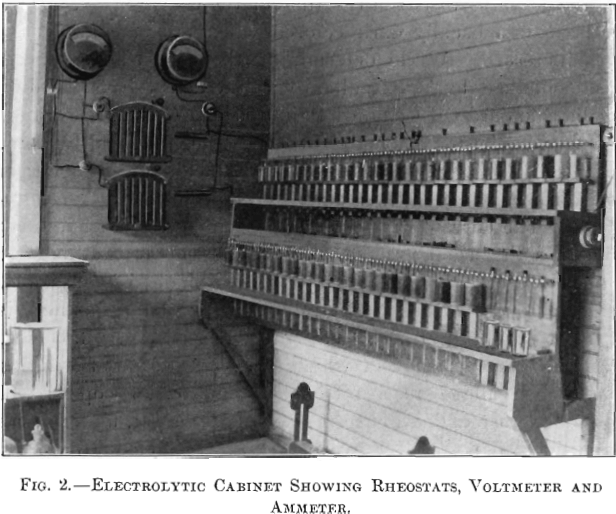
For weighing the pulp I use a weight of 855 mg., which has been christened a “ plum,” and multiples thereof, in order to avoid the necessity of calculating each result, the weight of peroxide found being called lead.
The same style of electrodes are eminently suited for copper work. Apart from the cost of platinum the great drawback in the use of the electrolytic method for copper-determination in daily work is the time required for the electrolysis, usually amounting to from 8 to 12 hours. Many substances were tried in the attempt to lessen the time of the copper-assay, but the cathode was usually dark colored when the current density was increased. While determining the copper in a concentrate obtained by Mr. Haultain with the use of No. 4 hard oil of the Standard Oil Co. and retaining some of the grease, a peculiar glossy luster was noticed on the deposited copper. Some of this hard oil was treated with nitric acid, and the solution, freed from grease, was then added to the solution to be electrolyzed. The current was increased to 5 amperes, which is about 25 times the usual practice, and still the copper continued to come down bright. The preparation of this “ nitro ” compound, called “ dope,” for use in electrolytic copper-determinations, is as follows:—hard oil is boiled with strong nitric acid, cooled, and the grease removed, which leaves a deep-red solution that, when added to a nitric-acid solution of copper, will permit the use of high current densities and yet, under these conditions, deposit all the copper pure and bright in 3 hr. Moreover, arsenic and antimony, which interfere seriously in ordinary electrolytic precipitation of copper, do not contaminate the deposit of copper, even if present in very large amounts. More than 3,000 copper-determinations have been made in this way, and experiments have also been made on smelter flue-dust and copper-dummies containing large amounts of arsenic and antimony. In these cases the results were always satisfactory.
The use of the oil in electrolytic work is as follows: the ore, weighed into tall battery-beakers of 100-cc. capacity, is digested with 7 cc. of nitric acid, and boiled until nitrous fumes have been expelled. About 2 cc. of dope, diluted with nitric acid, are added (or it may be added with the acid), the beaker, filled with water and allowed to settle for a moment, is then electrolyzed with a current of 1.5 amperes for 3 hr. The cathode must be sand-blasted, otherwise the copper is apt to fly off when it is ignited.
There should be no gassing whatever at the cathode during electrolysis. The cathodes frequently gas when the current is first turned on, but if turned off for a second, and then on, the gassing should cease. The cathode frequently gasses at the end of 3 hr., when the assay is finished.
Several different nitro-hydrocarbons have been tried instead of the dope in the 3-hr. copper-assay, and of these, di-nitro-alpha napthalene produces, with a little more care during electrolysis, results nearly equal to the dope.
