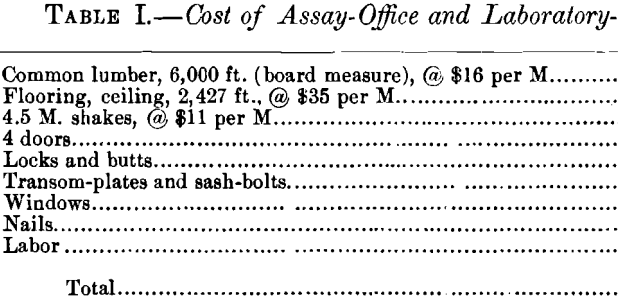The work of a chemist at a busy smelting-plant differs radically from the work of a student, who takes all possible care to have his analysis exact within hundredths of a per cent., and has plenty of time to devote to the operation. The works- chemist, having the shortest time in which to make his determinations, should have a laboratory arranged as conveniently as possible to save time in carrying out his work. By a conveniently-arranged laboratory, I do not mean an elaborately furnished one; but rooms suitably fitted up and supplied with the necessary apparatus to do the work expected from the chemist within the requirements of technical analyses.
It is not uncommon at large smelters to have the night assayer prepare 30 or more samples for titration, in time for the chemist to begin work on them at 7 o’clock on the following morning. During the time that the solutions are being heated on the hot-plate, the chemist is weighing out portions of slag- samples which have been taken from each furnace. As a rule, he determines the various percentages of silica, iron and lime in the slag (the values in lead and silver, and, sometimes, that of gold being determined by the assayer), but very frequently the slag is analyzed for other constituents as well; and every 14 days a complete analysis of it is made. The chemist cannot give his entire time to a single analysis until it is completed, for the reason that a dozen or more additional samples come in regularly which have to be analyzed for “ insolubles,” silica, iron, lime, zinc, sulphur, lead, copper, alumina, manganese, arsenic and antimony.
Every 14 days, both flue-dust and speiss are tested for their copper- and lead-contents, and occasionally a complete analysis of these products is required. The lead-bullion is analyzed for its content of iron, copper, arsenic, antimony and bismuth. In addition to the routine work mentioned above, an analysis of coal or coke for the percentage of ash, sulphur, volatile matter and fixed carbon, as well as a determination of its calorific value, is called for, and occasionally an analysis is made of refractory brick and of gas. The chemist, in order to do all this work must devote his entire time for 10 hours every day. The above-named conditions are not hypothetical, but have actually occurred in my own experience at a large lead-smelter; and I have taken them into consideration in my present design of laboratory.
The chief requisites for a works-laboratory are abundant light and good ventilation, because, even with the best arrangement of hoods and flues, the air is liable to become contaminated by acid fumes.
The laboratory-building is constructed of wood, and is ceiled inside with tongue-and-groove lumber, leaving a 4-in. air-space between the outer and inner walls. The roof terminates in a large ventilator, having windows swinging on pivots over the laboratory portion, while over the assay-office the ventilator has fixed shutters of 1- by 6-in. wooden slats, set at an angle of 45°, with 3-in. spaces between adjacent slats. A chemist’s hearth, having a hood, supplies the heat necessary to boil solutions, as well as for other uses. The main working-table is placed opposite the chemist’s hearth, which arrangement allows the chemist to carry on his analytical work and, at the same time, control the heating of the samples in sight on the hearth. The table is equipped on both sides with drawers, closets and shelves; the side nearest the hearth is used for quantitative work only, while on the other is a complete set of the reagents needed for qualitative mineral analysis. A small kerosene-stove, and a copper kettle, placed on the highest shelf of the table give a constant supply of hot distilled water. On opposite sides of the bottom of the kettle are two small bibbs, to which are fastened small rubber tubes, having at each outer end a pointed glass tube, and sufficiently long to reach both ends of the table. A Mohr pinch-cock regulates the flow of water to a nicety, and the 4-ft. head gives sufficient pressure to wash precipitates, filters, etc., thoroughly and quickly. This arrangement avoids the discomfort arising from continued inflation of the cheeks which the ordinary wash-bottle necessitates. A sink of glazed clay with drain-boards is placed at the end of the table.
A table opposite the main working-table is reserved for electrolytic analysis, and is equipped with an accumulator, which is used as the source of current. The accumulator is better than the common cells for the reason that it maintains a fairly constant potential. The current strength and potential are respectively measured by an ammeter having a range of 5 amperes with subdivisions of 0.1 ampere, and a voltmeter with a range of 10 volts, and subdivisions of 0.1 volt. The rheostat consists of a band of German silver placed in the circuit and having a sliding-contact which enables the operator to change the potential. As the electrolytic determinations are limited to copper- mattes, and occasionally leaf-copper for standardization-purposes, the amperage and voltage best suited to the chemical composition of the matte will be used; and when once ascertained, they will remain practically constant. Luckow’s cylinder and spiral are used as electrodes.
A table in front of a window is used for titrations and is provided with burettes, which can be filled automatically. This special type of burette consists of a 2-liter glass bottle with a pressure- and a delivery-tube passing through the cork, the delivery-tube connecting direct to the upper end of the burette.
The pressure necessary to force the solution from the bottle to the burette is supplied by two rubber bulbs having air-inlet valves so arranged that a continuous pressure may be produced. The inner (and larger) bulb is covered with a strong cord-net in order to remove the danger of bursting from too great a pressure. On the table are two closets, having shelves large enough to hold a 2-qt. bottle, and serving the purpose of keeping the solutions in the dark; the closet doors bear the symbols of the respective stock-solutions. In my laboratory I have the following-named stock-solutions,—potassium cyanide, potassium dichromate, potassium permanganate, potassium ferrocyanide, N/10 potassium hydroxide, N/10 sulphuric acid, copper sulphate, ammonium molybdate. An additional set of these solutions kept in reserve in the dark closet allows a convenient interval of time in which to prepare fresh solutions.
A cupboard is provided in which to store a complete set of the chemicals needed in this line of work, as well as such apparatus and glassware as are only occasionally used.
The balance-room is tightly ceiled, special care having been taken to make it dust-proof. It has three windows on each long side, and a swinging sash-door entrance at the end, which guards against collisions between assayer and chemist when passing through the door-way in opposite directions.
The bench on which the balances rest is supported by wooden posts set in concrete, and extending through the floor into the ground with a 0.25-in. clearance. The scales set on plate- glass, and when once adjusted, they remain level for a considerable time, any shrinkage of the table having no effect on the position of the plane of the plate-glass.
The general arrangement of the laboratory and assay-office is shown in Fig. 1.
The chemist’s hearth, a description of which may possess special interest to chemists and metallurgists at places where gas is not obtainable, is illustrated by Fig. 2. This hearth rests on the ground and the flue from it is filled with ashes and earth to within 18 in. of the hot-plate. In the front of the ash-pit, beneath the assay-office floor, is a 12- by 12-in. opening, which is closed with a piece of sheet-iron luted on with clay. The accumulated ashes in the ash-pit are removed through this opening, thus avoiding their removal through the assay-office. The ash-pit door shown in the drawing is used solely to regulate the draft. Old rails preferably are used as grate-bars.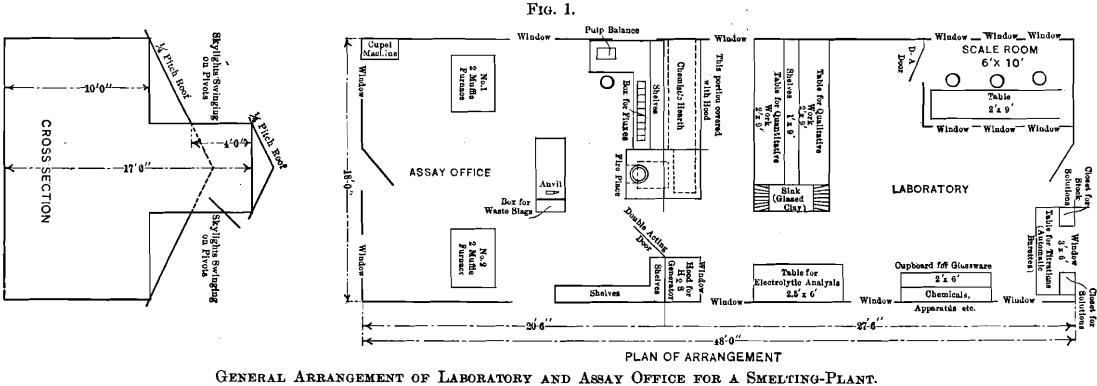
The front elevation of the hearth is shown in Fig. 2, which includes also the elevation of the fire-place front with the ash-pit door and the feed door. The lines, A B and C D, explain the respective elevations. The walls of the hearth consist of one course of brick, excepting at the stack, which is of a course and a half, and the fire-box, which is of two courses. These walls support the hot-plate having its upper surface 43 in. above the level of the floor. A detailed dimensioned drawing of the hot-plate is given in Fig. 4. The plate is cast in two pieces, having a lap, so that a tight joint may be obtained, and at given intervals ribs are cast as a safeguard against warping. A circular hole, over which the still is placed, is left in the plate. A portion of the hot-plate 2.5 ft. by 6 ft. 8 in. is covered with a hood, which rests on one layer of bricks, except at the hottest parts, where there are two layers in order to protect the wood. The back side of the hood does not rest on bricks, but is separated from the plate by a 2-in. air-space extending the entire length of 6 ft. 8 in. Access to the hot-plate is obtained through two windows, each having 12 lights of glass and hung on butts. The hood is tightly ceiled with tongue- and-groove lumber, and has an 18- by 18-in. wooden chimney, 10 ft. high, to carry off the fumes. The temperature of the inside of the hood and hood-chimney is sufficient to draw in fresh air constantly and thus improve the ventilation of the laboratory.
The great advantage of the hot-plate is its gradual decrease of temperature towards the chimney. The heating of solutions is generally started at the coolest place, and gradually continued toward the hottest part. The heat is diffused over a large area, and is not concentrated at one small spot as is the case with a Bunsen burner, and the boiling over of solutions is thus easily avoided at the expenditure of the least attention and care; thus allowing the chemist time in which to attend to other work. A small uncovered portion, 2.5 ft. by 3 ft. 4 in., is reserved for operations which are preferably conducted in the open air. The fireplace extends into the assay-office, and is closed off from the laboratory by a ceiled partition, formed by the continuation of the back side of the hood.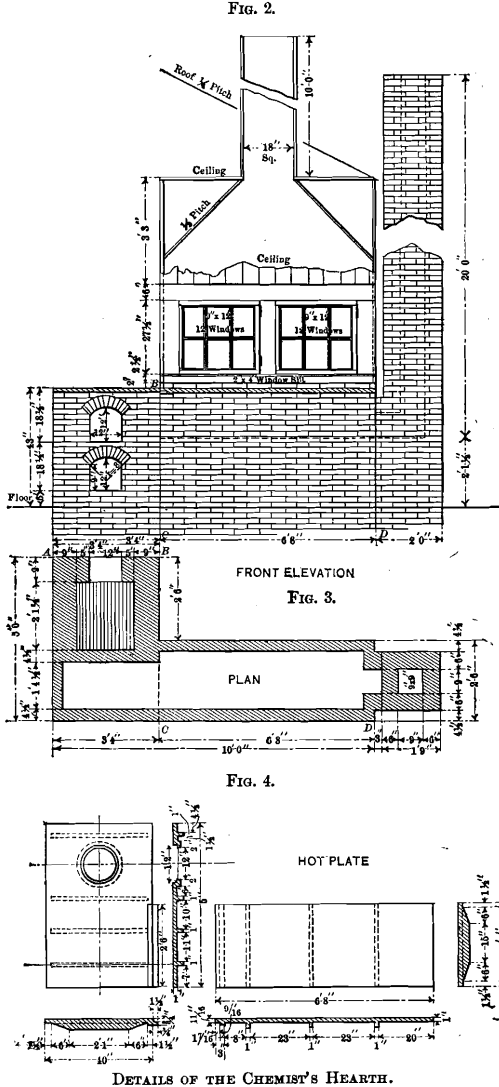
A still, placed over the circular hole described above, provides the laboratory with about 14 gal. of distilled water daily. The still now used is called the Cuprigraph Sanitary Still, No. 11, and when once regulated requires very little additional attention. The hearth thus serves two purposes, one, to provide the laboratory with distilled water; and the other, to give the chemist, at the same time, a very efficient way of heating solutions. The distillation of the water utilizes much of the heat, yet the hearth is necessarily wasteful, the flue being too short for economizing fuel. The weekly fuel-consumption, however, is only from one-half to three-quarters of a cord of wood, which, at $4 a cord, is equivalent to an operating-expense of from 28 to 42 cents a day.
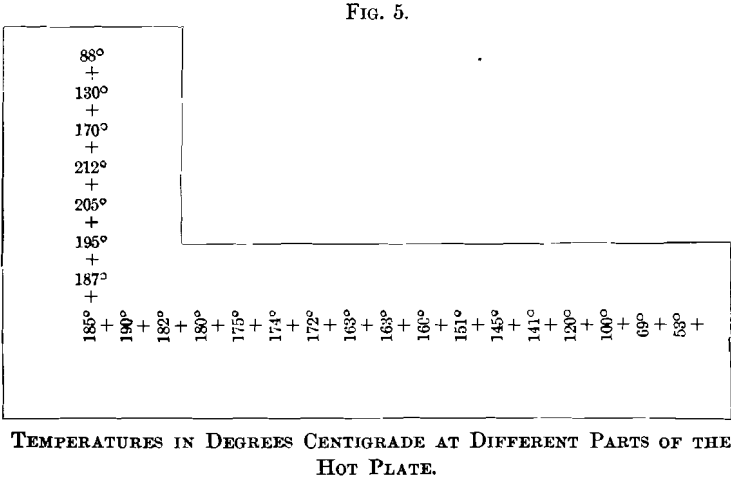
Fig. 5. shows the decrease in temperature with increasing distance from the fire-place. The temperatures were taken with a thermometer registering up to 250° C., and, while the readings are not exact, because the hearth was fired intermittently, they convey a fairly true idea of the temperature at different portions of the plate. Generally the hot plate is not used at temperatures as high as those recorded.
The laboratory is also provided with a hood, containing a hydrogen sulphide generator.
The assay-office has two muffle-furnaces, one kept as a reserve, or used when an accumulation of work has to be done. A general fluxing-mixture is kept, which, with slight alterations, can be used to flux ordinary ores.
All the samples are received from the sampling-mill in a ground condition, passed through a 100- or 125-mesh sieve. No grinding or sampling is done in the assay-office. The cupels are made by a cupel-machine.
The cost of the assay-office and laboratory-building (not including chemical apparatus and supplies) is given in Table I., and a detailed analysis of cost of chemist’s hearth.