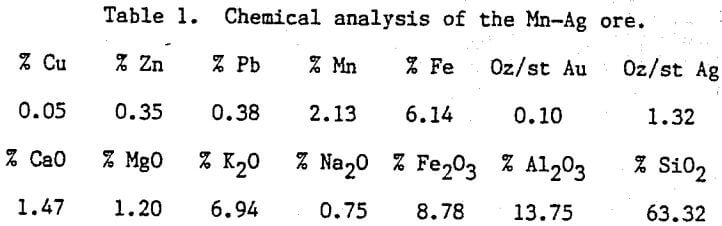
Table of Contents
Leaching Chemistry of Gold Silver and Manganese
Thiourea is a reducing and complexing agent and can be used for the processing of gold and silver. The redox potential of gold(I) and silver(I) thiourea complexes in acidic thiourea solutions are 0.380 and 0.023 V (vs SHE), respectively (Kazakov et al., 1983; Lodeishchikov et al., 1975). Thiourea, in the presence of an oxidant, oxidizes in successive stages to a number of products. The first stage is the formation of formamidine disulfide (NH2(NH)CSSC(NH)NH2) which is also an active oxidant for gold and silver dissolution. The redox potential of the thiourea/formamidine disulfide system is 0.42 V vs SHE (Preisler and Berger 1947) The oxidation of thiourea in the presence of an oxidant such as 02 could be represented as follows:
4NH2CSNH2 + O2 = 2NH2(NH)CSSC(NH)NH2 + 2 H2O,
E° = 0.81 V…………………………………………………………………………..(1)
Thiourea can be also used as a reducing agent for manganese in the processing of low-grade manganiferous ores. Thus, pyrolusite can be leached according to the following reaction:
2NH2CSNH2 + MnO2 + 2H+ = Mn²+ + NH2(NH)CSSC(NH)NH2 + 2H2O,
E° = 0.81 V……………………………………………………………………………(2)
Formamidine disulfide, produced by the above reaction, oxidizes gold and silver and forms bis-thiourea gold and tris-thiourea silver complexes as follows:
2Au + NH2(NH)CSSC(NH)NH2 + 2NH2CSNH2 + 2H+ = 2Au(NH2CSNH2)2+,
E° = 0.04 V……………………………………………………………………………….(3)
2Ag + NH2(NH)CSSC(NH)NH2 + 4NH2CSNH2 + 2H+ = 2Ag(NH2CSNH2)3+,
E° = 0.395 V……………………………………………………………………………………….(4)
Leaching Experiments
Reagent grade chemicals and distilled water were used in all experiments. Sodium bisulfite, NaHSO3, was used as the source of SO2 for leaching of manganese minerals in SO2 pre-leaching experiments. Agitation leaching experiments were carried out in a multi-neck glass reactor placed in a water bath at constant temperature. At the beginning of each experiment 4 the reactor was charged with 500 ml of test solution containing a specific concentration of sulfuric acid, thiourea, and 50 grams of ground ore. At predetermined times a 10 ml solution sample was removed for analysis. Cyanide leaching experiments were carried in the presence of 0.02 M NaCN and 0.02 M NaOH using the washed residue from the thiourea leaching or SO2 leaching experiments.
Results and Discussion
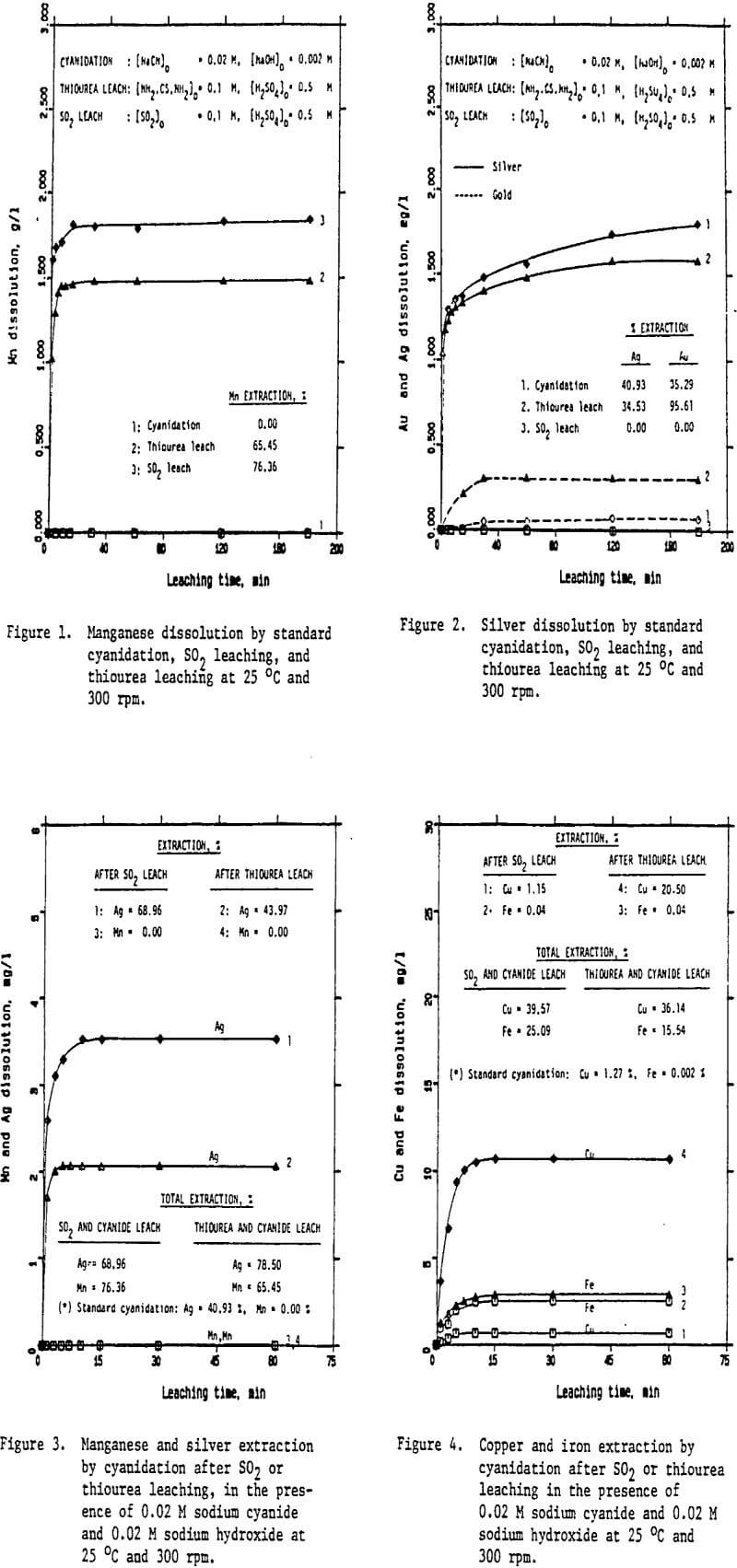
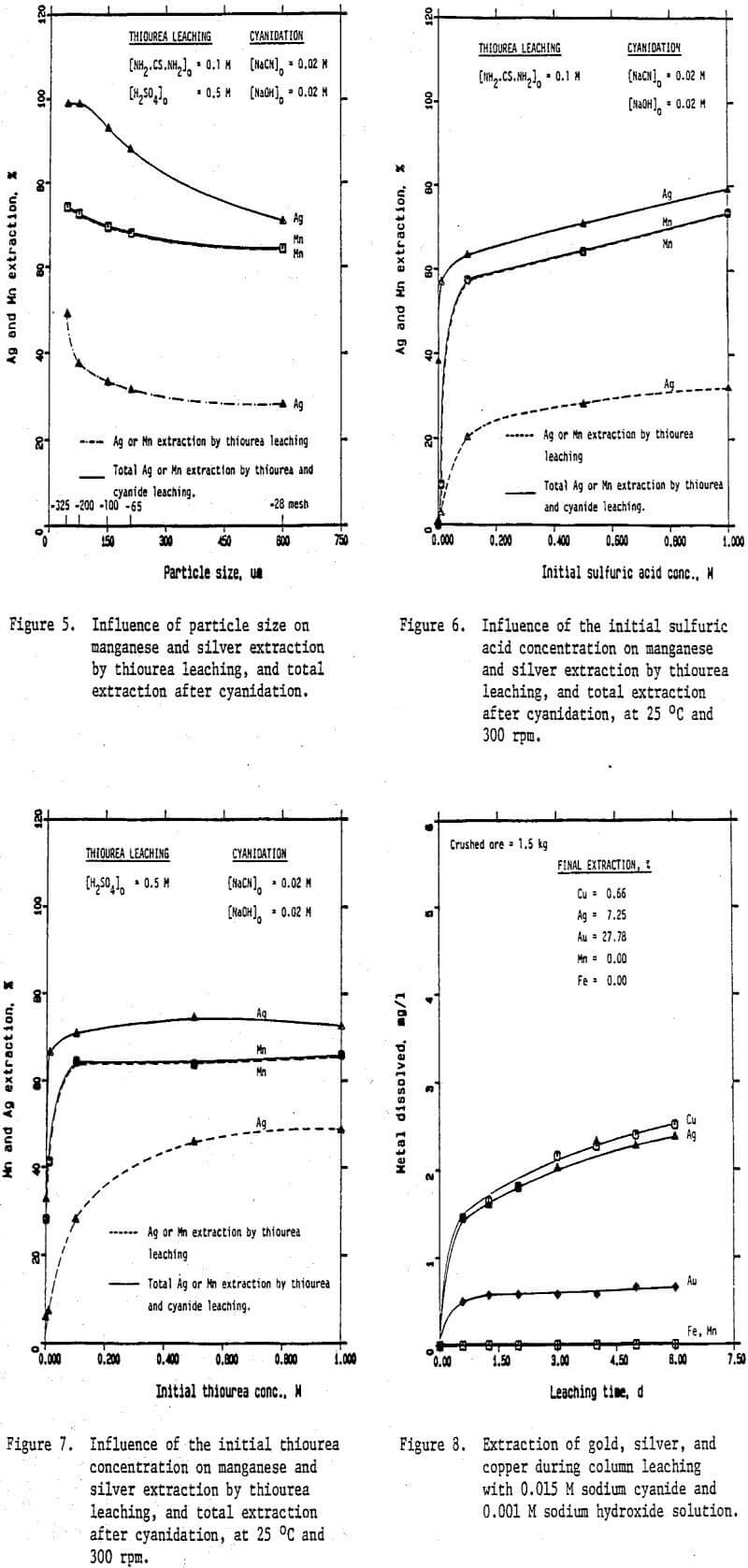
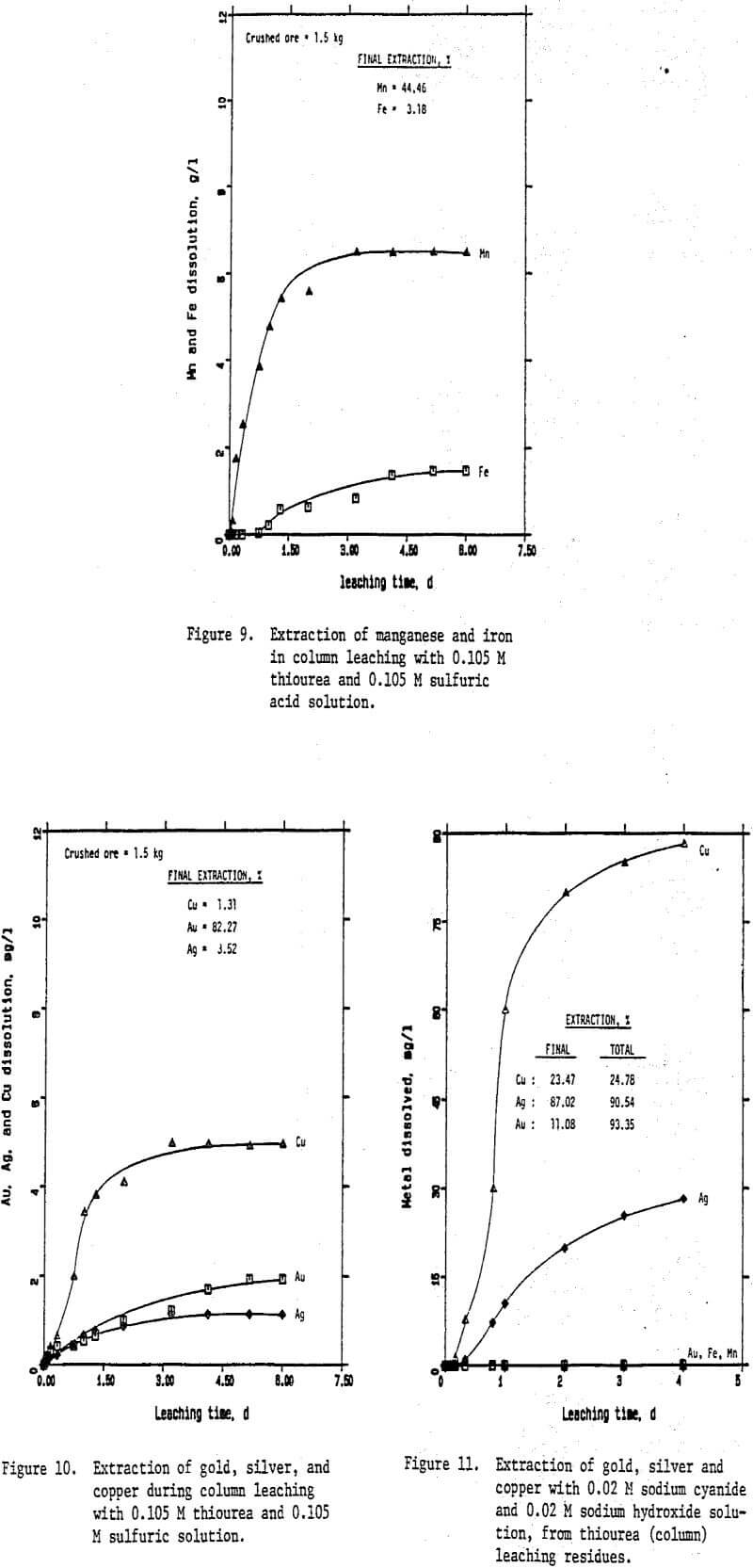
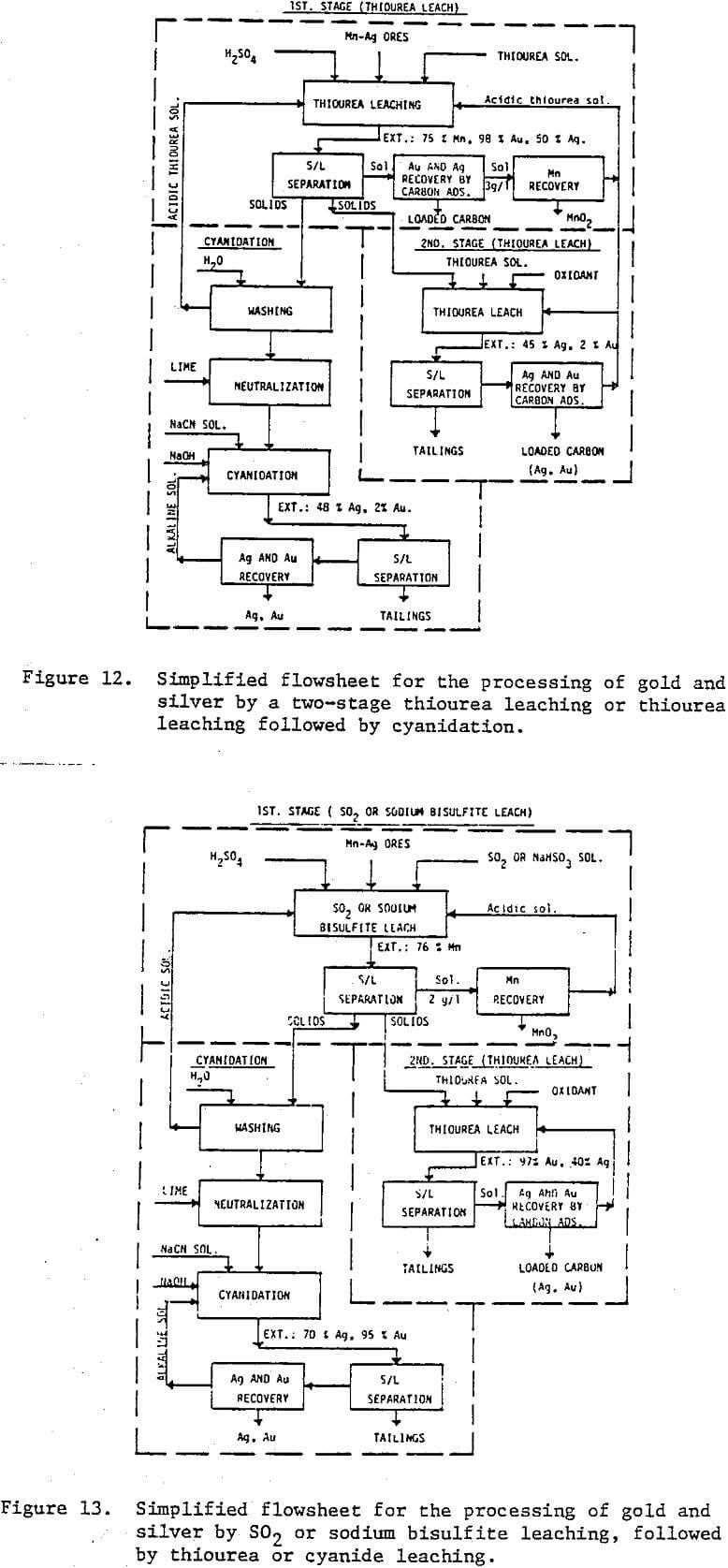
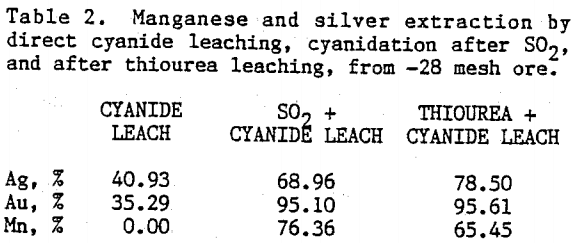
Preliminary experiments were carried out on silver extraction from the Mn-Ag ore to compare the SO2, thiourea, and cyanide leaching processes by agitation leaching, using -28 mesh material. Temperature and agitation speed were maintained at 25°C and 300 rpm, respectively.
Influence of Sulfuric Acid Concentration
The influence of sulfuric acid concentration on thiourea leaching was studied in 0.0 to 1.0 M solutions in the presence of 0.1 M thiourea. Cyanidation of the washed residues from thiourea leaching experiments were carried out in 0.02 M sodium cyanide and 0.02 M sodium hydroxide solutions. Figure 6 presents results on manganese and silver dissolution. Recoveries of manganese and silver by thiourea leaching, in the presence of 1.0 M sulfuric acid, were 73.60 % and 32.26 % , respectively. Total extraction of silver after cyanidation was 79.25%. Silver and manganese dissolution increased with increasing sulfuric acid concentration during thiourea leaching but they level off at about 0.2 M sulfuric acid concentration.
Influence of Thiourea Concentration
The influence of thiourea was studied om 0.0 to 1.0 M solutions in the presence of 0.5 M sulfuric acid. Cyanidation of the washed residues from thiourea leaching experiments was carried out in 0.02 M sodium cyanide and 0.02 M sodium hydroxide at 300 rpm.
The thiourea consumption will be based mostly on the amount of MnO2 present in the ore. Thus, assuming all manganese were in the form of MnO2, the theoretical thiourea consumption, according to Equation 2, will be on the order of 59 kg/t ore. However, the experimental results indicated that the thiourea consumption was about 27 kg/t ore. Low thiourea consumption also suggests that the manganese in the ore is mostly present in the form of complex oxides.
A Two-Stage Thiourea Leaching
The first stage thiourea leaching was carried out without oxidant under optimum experimental conditions at 0.169-0.2 M thiourea and 0.165-0.2 M sulfuric acid. After one hour of leaching time about 50 % silver and 98 % gold were solubilized. The residue from the first stage was subjected to thiourea leaching in the presence of 0.052 M ferric sulfate to extract the undissolved silver. Extractions of 12.41 % silver and 1.55 % gold were realized after 15 minutes of leaching time. The final extractions of gold and silver was 45 % Ag and 2 % Au after one hour of leaching. If the ore is lean in silver, however, the second stage leaching may be not be feasible. It should also be pointed out that leach solutions from the first stage of thiourea leaching contains approximately 3 g/l manganese.
SO2 Leaching Followed by Thiourea Leaching
Sulfur dioxide leaching was carried out using 0.1 M sodium bisulfite and 0.105 M sulfuric acid for one hour. Manganese was selectively dissolved, gold and silver were then extracted from the leach residues by thiourea leaching, in the presence of 0.026 M ferric sulfate and 0.105 M thiourea.
It is evident that SO2 preleaching will result in substantial savings in thiourea consumption. Manganese can be recovered from SO2 leach solutions as MnO2 by oxidation and precipitation. Precious metals can be recovered from thiourea leach solutions by carbon adsorption. The final acidic thiourea solution can be recirculated to the thiourea leaching circuit. A simplified flowsheet is also presented in Figure 13 for this process (Zegarra, 1987).