To understand interactions between physical and chemical variables, a synthesis rather than an analytical approach is needed. In analytical approach, sub-processes of a system are isolated to determine the cause and effect relationships between performance of the sub-process and the system variables, whereas in synthesis approach a global view is taken to identify various interactions.
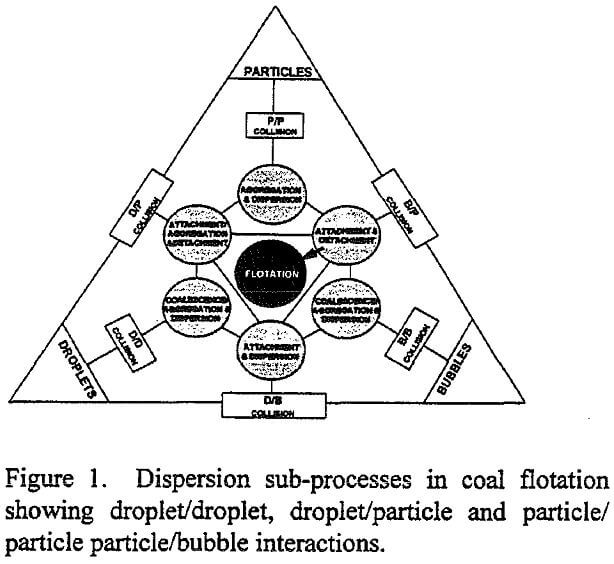
A schematic representation of the various sub-processes which take place in the pulp phase is given in Figure 1. The figure is drawn specifically for coal flotation, although a similar scheme can be easily conceived for other flotation systems. One can readily see that three major components, namely, coal particles, oil droplets and air bubbles, participate in many sub-processes in the flotation pulp, contributing to non-linear cause and effect relationships. Water is not included in the figure since it is the continuous medium in which particles, droplets and bubbles interact. The interactions could occur simultaneously or sequentially depending on the mode of reagent addition, and absence or presence of a separate conditioning stage prior to air addition. The variability in properties such as size distribution, concentration, physical or chemical composition, is not shown for brevity, but their effect on each sub-process is recognized. Among the sub-processes illustrated in the figure only those which involve particles and bubbles have been studied thoroughly. The sub-processes involving droplets and particles, and their influence on the kinetics or selectivity of flotation are poorly understood, however. If a variable affects more than one sub-process, interaction between variables arise. To study such interactions, the effect of the variable on all sub-processes must be known, which is a formidable task because it requires a knowledge of all the sub-systems and interactions among them.
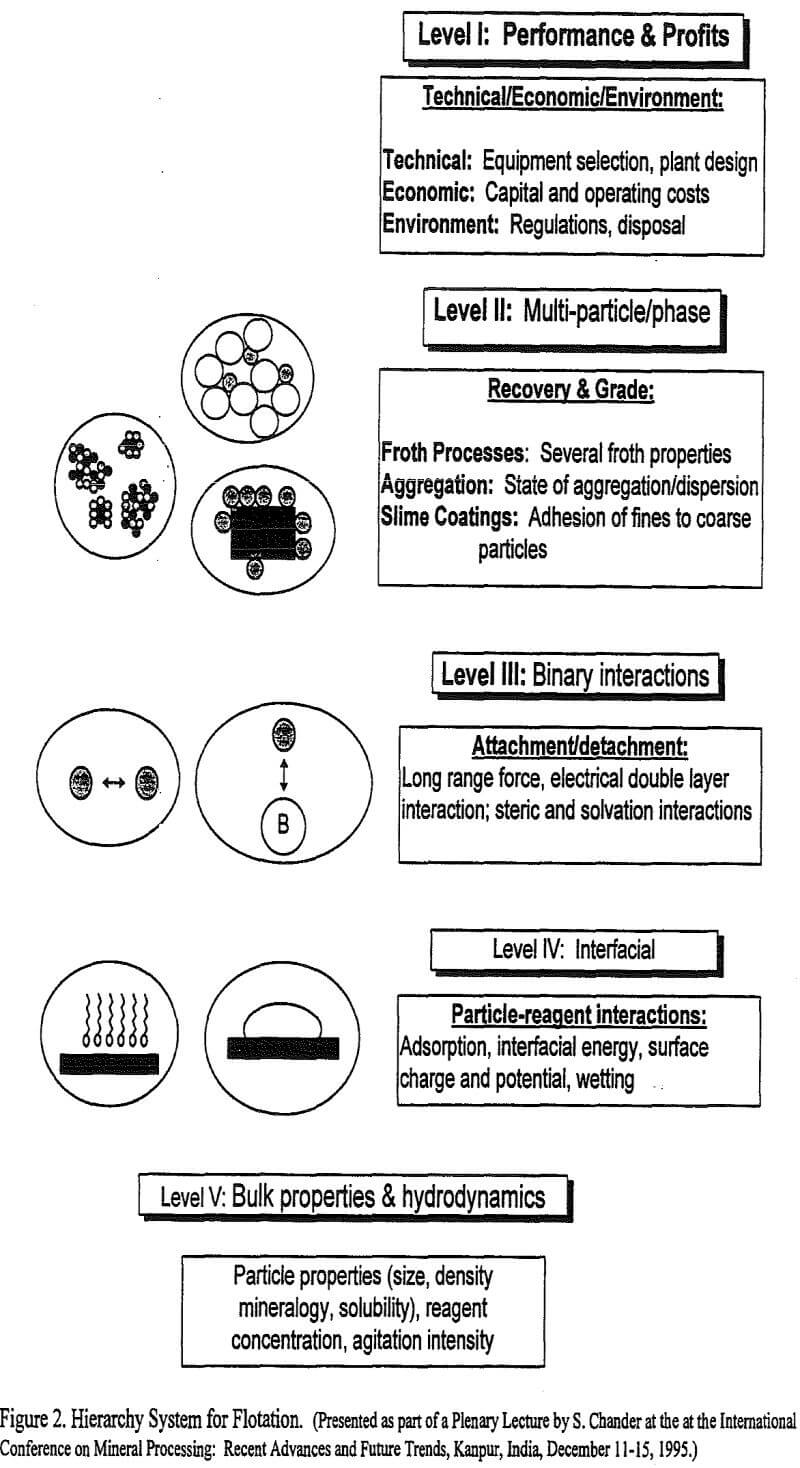
A hierarchy system, presented in Figure 2, is proposed in this article to rationalize the various type of interactions that occur in a flotation system. The importance of such an integrated approach to understand the system behavior becomes clear if one is interested in determining and predicting the effect of both the physical variables, such as rate of mixing, particle size, particle density, and the chemical variables, such as reagent type and concentration, on flotation performance. The performance and profits depend upon ore grade and reserves, plant design, environment regulations and waste disposal issues, which are referred to as Level I criteria in Figure 2. The Level I parameters are determined by, as well as they depend on operating conditions and performance in the flotation cells. These are referred to as Level II parameters in Figure 2. The recovery and grade in turn depend on a large number of sub-processes shown in Figure 1 and could be represented by Level III and IV parameters in the hierarchy system presented in Figure 2. The effect of changes in the above variables becomes complex because different variables effect various sub-processes in disparate ways. To control the performance and profits at Level I, for example, one may have to adjust the particle size distribution, reagent type and concentration or agitation intensity, which are Level V parameters. Changes in Level V parameters will affect interfacial interactions at Level IV, binary interactions at Level III, and multi-phase interactions at Level II. Thus, the interactions at a lower level affect properties of the system at higher levels in an orderly manner.
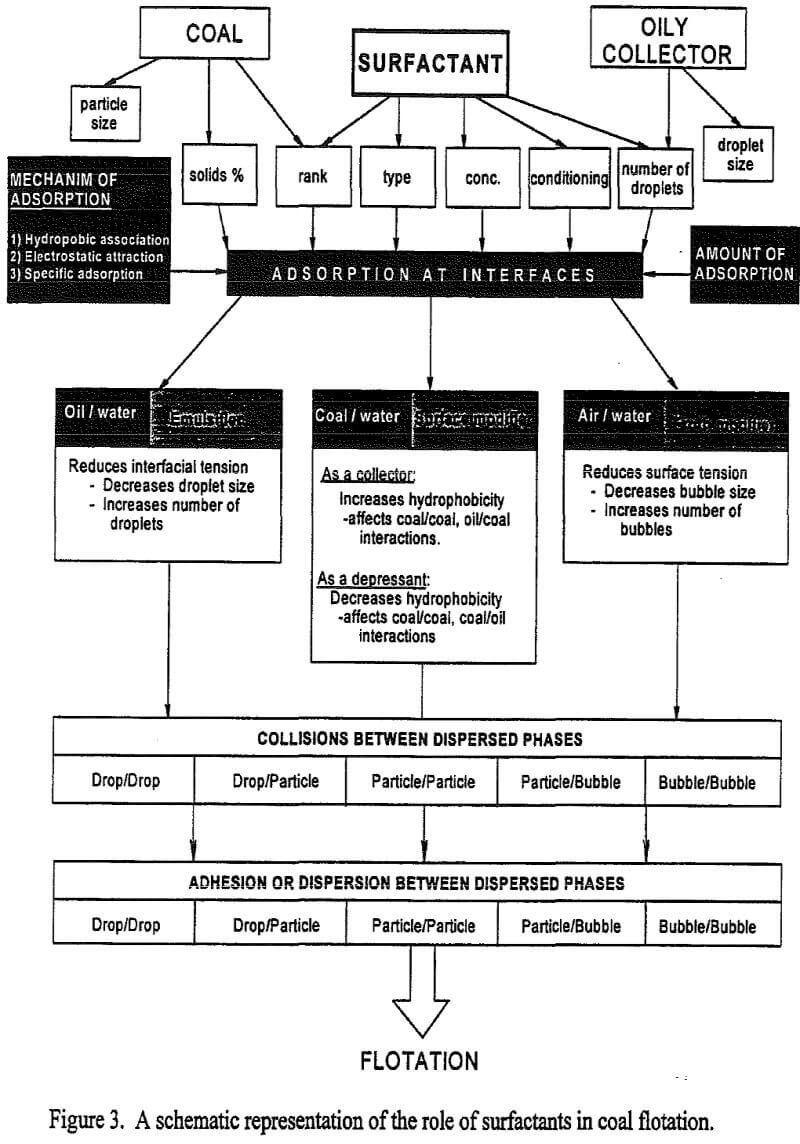
Flotation of Bituminous Coals
Two coals, namely Indiana VII and Illinois No. 6, were investigated in this part of the study. These coals were selected because of their low hydrophobicity and a need to use large amount of oily collector.
Three sets of experiments were carried out with the Indiana VII coal sample to determine the effect of PPO/PEO (polypropylene/polyethelene) block co-polymers on flotation. In the first set of studies, dodecane was used without a block co-polymer in a concentration range between 0.35 to 11.2 kg/T to obtain a satisfactory recovery (Figure 5). The recovery of clean coal was low and the ash rejection was poor. After 2 minutes of flotation, a clean coal with an ash content of 5.0% and a combustible matter recovery of only 22% was obtained with the highest dodecane concentration (11.2 kg/T) tested. In the second set, the dodecane concentration was kept constant at 11.2 kg/T, and the surfactant concentration was varied between zero to 4.4 x 10 -4 M. The results of these studies are presented in Figure 6. Addition of surfactant increased both the ash rejection and the combustible matter recovery. A clean coal recovery of 94% with an ash content of 3.75% could be obtained at the highest surfactant concentration tested (4.4 x 10 -4 M). It is seen from figure 6c that at low concentrations, Pluronic 25R- 1 (a reagent with PPO/PEO/PPO configuration) was more effective in increasing the combustible matter recovery and in decreasing the ash compared to the Pluronic L-64. The lowest ash for this coal was obtained at a concentration of 5.5 x 10 -5 M in the presence of Pluronic 25R-1.
In the third set, dodecane concentration was also varied in addition to surfactant concentration to determine the dependence of surfactant effect on dodecane amount. Figure 7 shows the dependence of the clean coal recovery and ash content on the amounts of dodecane and surfactant. It can be seen from the figure that both the combustible matter recovery and the ash rejection increased with surfactant and dodecane concentrations. At 2.8 kg/T of dodecane, increasing surfactant concentration had negligible effect on the overall flotation response. At 5.6 kg/T of dodecane, the effect of surfactant was more. At 11.2 kg/T of dodecane, the degree of ash rejection started to level off while the recovery of clean coal continued to increase. The ash content of clean coal for the best condition obtained at this dodecane concentration was slightly higher than the ash content obtained in the case of 5.6 kg/T of dodecane. That is, the effect of the same amount of surfactant on the flotation response was different for different dodecane concentrations.

