Table of Contents
This standard prescribes the cupellation or fire assay method for assaying of gold in gold bullion, gold alloys, and gold jewellery and artefacts covered in IS 1417 “How to Assay Gold” Procedure.
The following standards contain provisions which, through reference in this text, constitute provisions of this standard. At the time of publication, the edition indicated was valid. All standard are subject to revision and parties to agreements based on this standard are encouraged to investigate the possibility of applying the most recent edition of the standards indicated below:
 For the purpose of this standard, the following definitions shall apply.
For the purpose of this standard, the following definitions shall apply.
3.1 Bullion — Precious metals in the form of bar, ingot, plate, etc (refined or unrefined).
3.2 Cupellation — An oxidizing fusion of lead, gold, silver and associated base metals in a cupel which absorbs the lead oxide along with base metals oxides leaving a bead of gold and silver (along with platinum group metals, if any) on the cupel. 
3.3 Fine Gold — It is gold having fineness 999 parts per thousand (%o) and above without any negative tolerance and free from Pb, Hg, Bi, Se, Te and platinum group metals.
3.4 Inquartation — The addition of silver to an assay sample to facilitate parting.
3.5 Parting — Separating silver from gold by selectively dissolving the silver in acid, usually nitric acid.
The gold alloys are inquarted with silver, compounded with lead and cupelled in a cupellation furnace until a precious metal button is obtained. After flattening and rolling, the silver is extracted (parted) in nitric acid and the gold weighed. Possible systematic errors in the procedure are eliminated by assaying standard proof samples in parallel.
- White gold alloys containing palladium and/or nickel as well as alloys with 990 or more parts per thousand (%o) of gold require some procedural changes.
- If the bullion contains any of the platinum group metals, the accuracy and precision cannot be achieved.
5 SAMPLING
5.1 Sampling by Cutting or Drilling
5.1.1 When the bullion is in the form of an ingot or slab and is of high fineness, sampling by ‘cutting’ or ‘drilling’ may be adopted.
5.1.2 Cutting Method
Samples are cut from diagonally opposite corners of the upper and lower sides of the bar. Cutting is to be done with a sharp narrow steel chisel. From each cut, 1 to 1.5 g sample may be taken. The cut pieces should be flattened, rolled, cut into small pieces and thoroughly mixed together.
5.1.3 Drilling Method
The ingot is drilled on the top corner and on the opposite bottom corner with a 3 to 10 mm high-speed tool steel drill. When it is known that ingot is not of uniform composition or is of low-fineness, the minimum 2 drillings should be taken from the top and 2 from the bottom at diagonally opposite corners. The surface drillings resulting from the first few revolutions of the drill, should be rejected till the hole on the ingot is equal to the diameter of the drill. The drilling should be carried out to a little more than half-way through the ingot. The minimum weight of drillings from the above 4 borings should not be less than 5 g. In case of fine gold the drillings should be cut into small pieces and mixed thoroughly before taking up for assay. In case of low-fineness samples, 4 lots of drillings may be assayed separately or 2 lots from the top may be mixed to form one sample and 2 lots from the bottom mixed for a second sample. When the material is in the form of sheet or wire, small pieces shall be cut preferably from opposite ends and thoroughly mixed together.
- In case the sample is found to be non-homogeneous by assaying, the method as given in 5.2 should be adopted for sampling
- Sampling for assaying jewellery shall be done by scrapping or by cutting after removing the surface colour/coating or as agreed upon
- When the composition of the samples is unknown, use a preliminary assay for the estimation of the fineness of gold For the distinction between palladium and nickel white golds, the touchstone test can also be used. If the cornet breaks up during the parting process, this is often an indication of excess silver
- Sampling procedure for jewellery shall be agreed upon until a corresponding standard method is published
5.2 Dip Sampling
When the bullion/jewellery has been melted it should be stirred well with a preheated graphite stirrer and a dip sample of about 5 g taken with a preheated graphite sampler from the molten metal from the middle of the pot just before casting. The sample may be granulated or cast into a small button or allowed to solidify in the sampler. This may be rolled and cut into small pieces for assaying. The granules may be hammered, if necessary, for complete removal of trapped water. Alternatively quartz tube may also be used for drawing dip sample.
- In case of dispute with regard to sampling of ingot, slab, bar, sheet or wire or jewellery a ‘dip’ sample shall be taken from the representative lot.
- Granulation of the sample is not satisfactory for bullion of low-fineness as during pouring of the molten metal into water some of the base metals present may oxidize, thus altering the composition of the sample
- Before taking samples by any of the above methods, all surface dirt from the article shall be removed and all instruments for cutting, scrapping, hammering, etc. shall be cleaned thoroughly. No oil shall be used for drilling
- When the bar has been pickled by dipping in acid after casting, the surface shall be well scrapped before sampling The first portion of metal removed during the preparation of the sample shall be rejected
6 REAGENTS
6.1 Unless specified otherwise, pure chemicals and distilled water (see IS 1070) or water of equivalent purity free from halides and suspended impurities shall be employed in the tests.
NOTE — ‘Pure chemicals’ shall mean chemicals that do not contain impurities which affect the results of analysis
6.2 Borax (Na2B4O7), anhydrous.
6.3 Pure Gold for Proof Samples
Gold of minimum purity 999.9 parts per thousand (%o) by mass.
6.4 Copper Foil/Wire or Disc
Minimum purity of 999 parts per thousand (%o) by mass and free from gold and platinum group metals.
6.5 Lead Foil
Lead, foil and beads, assay grade minimum purity 999 parts per thousands by mass (%o) and free from gold, bismuth and platinum group metals.
6.6 Nickel
Minimum purity 999 parts per thousand (%o) by mass, free of gold and platinum group metals.
6.7 Palladium
Minimum purity 999.9 parts per thousand (%o) by mass, free of gold and platinum group metals.
6.8 Parting Acid No. 1
Dilute nitric acid specific gravity 1.2 (g/cm³) free from halides and suspended matter.
6.9 Parting Acid No. 2
Dilute nitric acid specific gravity 1.3 (g/cm³) free from halides and suspended matter.
6.10 Silver
Silver of fineness 999 (%o), Min and free from gold and platinum group metals.
NOTE — The presence of elements beyond three decimals of purity may be considered as freedom from purity
7 ASSAY BALANCE
7.1 It is a balance with sensitivity 0.002 mg for assaying gold sample up to 990 fineness. For assaying of gold alloys above 990 fineness, the sensitivity 0.001 mg is required.
7.2 Assay Cleaning Brush
It is a brush with stiff bristles or nylon but not brass.
7.3 Balling Pliers
A pair of steel pliers with smooth concave chops, the diameter of the cavity in the chops being about 10 mm.
7.4 Cupels
Made of calcium phosphate or magnesia usually of diameter 16 mm to absorb 4 g lead, 22 mm to absorb 6 g lead or 26 mm to absorb 10 g lead or block of cupels of similar absorbance.
7.5 Cupellation Furnace
It is muffle type furnace provided with inlets and outlets for air for maintaining an oxidizing atmosphere constantly during operation. The muffle shall be capable of being heated uniformly up to a temperature of I 150°C.
7.6 Hammer and Anvil
These shall have bright and clean striking faces. The hammer shall have a minimum mass of 400 g. This may be replaced by a press, polished and reserved for this purpose.
7.7 Parting Flasks or Platinum Basket
7.8 Parting Tray
A number of small thimble like perforated cups of Pt or Pt/Ir or Pt/Rh or unglazed silica supported in a frame of the same material.
7.9 Rolling Mills
A small jewellers rolling mill.
7.10 Scorification Dishes
Usually of diameter 50 mm.
7.11 Tongs and Forceps
Of various forms for changing the cupels and handling assay-pieces, etc.
7.12 Trays
Of various forms for keeping the assay-pieces, buttons, rolls, fillets, cornets, etc.
PROCEDURE FOR ASSAYING GOLD IN GOLD BULLION
8.1 Weighing of Sample
Weigh at least two samples of the alloy each of 500 mg to an accuracy of 0.002 mg. If sample mass is insufficient, take preferably 250 mg (in duplicate) weighing to the same accuracy. In case of white gold containing nickel, take 250 mg sample in duplicate. Run a proof assay in duplicate adding same quantity of nickel as present in the test sample.
The mass of lead foil (or foil + lead beads) should be 8 g (approximately) for sample of 500 mg and 4 g for 250 mg sample. In case of white gold, containing nickel, effective cupellation requires an addition of 4 g of lead, larger cupels and additional time for completion. In case of 375 and 585 carat gold, take 250 mg sample in duplicate and extra amount of lead 4 g and 2 g respectively towards the end of cupellation and allow sufficient time for completion of cupellation. Proof assays are also to be treated in the same manner.
8.2 Preparation of the Assay-Piece
8.2.1 In making up the assay-piece add enough silver to the sample so as to make up the total silver content between 2.3 and 3.0 times the mass of gold present in the sample allowing for the silver already present in the sample.
8.2.2 Weigh as in 8.1 at least two proof assay samples of proof gold (see 6.3) and pure silver in masses which correspond to the expected gold and silver content (including inquartation addition) of the assay sample. The total content of base metals in the assay samples is taken into consideration by addition of corresponding quantity of copper (see 6.4).
8.2.3 Wrap the weighed sample together with the required amount of silver and copper in the lead-foil and squeeze them in the balling pliers to form small balls. The assay- piece shall now be considered ready for charging in the cupellation furnace.
8.3 Preliminary Assay
If the approximate composition of the sample of gold alloy is not known, it is necessary to make a preliminary assay. For this purpose, weigh 2 lots of 100 mg of the alloy, add 300 mg of silver to one lot and then wrap each lot separately in 3 g of lead. Cupel both side by side . Flatten the button containing the added silver and boil in 15 ml of parting acid No. 2 for 15 min, wash the resulting gold, dry and heat to redness and weigh. The weight in milligrams gives directly the percentage of gold, and the weight in milligrams of other button gives the percentage of gold and silver together, the difference between the two gives the percentage of silver. The remainder will be base metal. When the approximate composition of the alloy has been ascertained, determine the amount of silver to be added for assay. Alternatively, preliminary assay can be done by other method such as XRF, ICP, AAS, etc.
8.4 Cupellation
Arrange the cupels carefully in the muffle furnace, preferably on a removable tray. When the furnace temperature is of about I 050 – I 100°C. place in them the assay-pieces and the proof assay samples each in its proper cupel, by means of a pair of long cupellation tongs. The charging should be done carefully, but as rapidly as possible, so as not to cool the muffle furnace unduly. Alternatively mechanical device may be used for charging all the samples at a time. Close the muffle door and allow the cupellation to proceed for 20 to 30 min depending on the amount of lead used, the temperature being raised to about I 100°C towards the end. The end of the cupellation is shown by the appearance of bright globules of gold-siiver alloy. The cupels may be withdrawn while the temperature comes down to approximately 850°C in the furnace till the buttons are solidified and then removed from the furnace.
8.5 Preparing the Assay-Piece for Parting
8.5.1 Remove the buttons from the cupels by means of pair of forceps and clean with a stiff brush. Flatten the buttons on a polished anvil with a polished hammer. Anneal the flattened buttons at about 700°C and pass in succession through the rolls to form elongated fillets of thickness 0.22 to 0.25 mm and number them serially. After being rolled, anneal again to soften them and then separately roll up between the finger and thumb into a cornet or spiral; making the lower side of the button the outer face of the cornet.
8.5.2 Place the cornets in the respective cups of the parting tray and immerse the entire tray in parting acid No. 1 at a temperature of 90-95°C and boil for 25 to 30 min or until no more nitrous fumes are observed. Take out the tray and drain from acid liquor, then wash by dipping in and out of a vessel of hot distilled water, drain again and immerse in a second lot of boiling parting acid No. 2. Boil the cornets in this way for 20 to 25 min, drain and wash thoroughly till the washing is free from silver nitrate. Dry the tray with the cornets by gently heating and then anneal in a muffle furnace heated to 800°C for about 5 min.
8.6 Proof Assays
The proof samples shall be subjected to the same operations side by side and under identical conditions with the assay-pieces. The number of proof samples shall be not less than two in each group of assay and shall be positioned evenly in the group.
8.7 Expression of Results
8.7.1 Method of Calculation
Calculate the gold content, W in parts per thousand (%o) by mass, of the alloy using the formula;
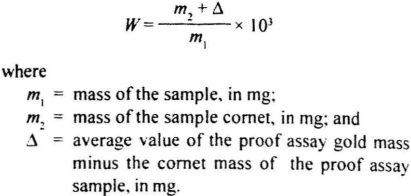
8.7.2 Repeatability
Duplicate determination shall give results differing by maximum 0.5 parts per thousand (%o) by mass for yellow and red gold alloys, maximum 1.0 part per thousand (%o) by mass for white gold alloys and maximum 0.2 parts per thousand (%o) for gold alloys containing 990 part per thousands or more gold. If the difference is greater than this, the assay shall be repeated. When analyzing alloys with a fine gold content of 990 (%o) or more, the values of for the proof samples (see 8.6) which run in parallel shall not differ by more than 0.04 mg. If the difference is greater than this, the assay shall be repeated.
PROCEDURE FOR ASSAYING GOLD IN JEWELLERY
9.1 Yellow Gold Alloys
9.1.1 Analysis Samples
Transfer at least two samples of the alloy, preferably between 125 mg and 250 mg weighed to the nearest ± 0.01 mg, into assay-grade lead-foil. The mass of the foil (or foil + beads) should be at least 4 g for yellow gold samples up to 200 mg, and 6 g for samples from 201 mg to 300 mg (250 mg). Add pure silver equivalent to 2.3 to 3 times the mass of fine gold present. Roll and compress the lead foil into a tight ball.
9.1.2 Proof Assay Samples
Weigh, as in 9.1.1 at least two proof assay samples of proof gold and pure silver in masses which correspond to the expected gold and silver contents (including the inquartation addition) of the assay sample. The total content of base metals in the assay samples is taken into consideration by the addition of a corresponding quantity of copper. Treat the proof assay samples and the assay samples in 9.1.2 and 9.1.1 in the same manner.
9.1.3 Cupellation and Treatment of Precious Metal Buttons
Place the assay and the proof assay samples (see 9.1.2) tightly wrapped in lead foil, on magnesium oxide cupels which have been preheated to at least I 100°C in the cupellation furnace.
Place the cupels with the proof assay samples as close as possible to the corresponding assay samples in the cupellation furnace maintained at I 050°C-I 100°C. Continue heating until this stage is completed (about 25 min) under oxidizing conditions. Remove the cupels from the furnace. Allow the precious metal buttons to cool down before lifting them from the cupels with the assay pliers. Squeeze the buttons and brush their undersides carefully with a brush to remove any adhering cupel material. Flatten the beads on the polished anvil with a polished hammer and anneal by heating just to red heat 700°C.
Roll them into 0.12 to 0.15 mm thick strips and anneal again. Roll the strips into cornets without contamination or loss of gold.
It may be noted that the cupel should be examined carefully to ensure that the precious metal bead contains all the sample gold. Small droplet residues indicate the need for a repeat determination in a smaller cupel.
9.1.4 Parting of the Silver/Gold Samples
Place the precious metal cornets in parting flasks. First immerse the comet in 20 ml of nitric acid (see 6.8) at a temperature at least 5°C below the boiling point and bring to the boil.
Continue heating for 15 min or until the evolution of nitrous fumes has ceased, whichever is longer, decant, wash with warm water and immerse in 20 ml of nitric acid (see 6.9) and boil for 10 min. Decant and wash the gold cornet with warm water at 60° to 70°C until it is free of silver nitrate. Transfer the gold cornets to small porous parting cups (porcelain crucible). Dry them and anneal at 700° to 750°C for about 5 min. Cool, and then weigh the gold cornet.
When assaying a series of samples of similar composition, instead of using a number of parting flasks the precious metal cornet can be parted with the aid of a dissolution basket consisting of Pt/lr or Pt/Rh which is equipped with a number of quartz, Pt/lr or Pt/Rh thimbles with perforated bottoms. The cornets are placed in the thimbles and basket is lowered slowly into nitric acid (see 6.8) at about 90°C. The acid is brought to the boil and allowed to boil gently for 15 min or until the evolution of nitrous fumes has ceased, whichever is longer. Remove the basket from the acid, rinse in water repeat the treatment in a second batch of nitric acid (see 6.9) and boil again for 15 min.
Remove the basket from the acid, rinse it with warm (60° to 70°C) water until it is completely free of silver nitrate and allow to dry. Finally place the basket with the gold samples for about 5 min in muffle furnace, heated to approximately 700° to 750°C. After cooling, the gold samples shall be weighed.
CAUTION — For the parting operations with nitric acid, a fume hood should be kept clean and used exclusively for this determination
9.2 White Gold Alloy Containing Nickel
If nickel is present, two equivalent variations on the method specified in this standard are acceptable. These involve either the use of additional lead or scorification.
9.2.1 Cupellation with Additional Lead
It is difficult to extract all the nickel in the alloy into the cupel by using the standard quantity of lead. Effective cupellation requires an additional 4 g of lead and the use of larger cupels. This extra lead may be incorporated at the start of the test, if the cupel is large enough to contain the increased volume of melt. Alternatively a button of lead is added to the hot precious metal bead in the cupel after the lead oxide fumes from the initial operation have ceased. Care is needed if the cupellation furnace is not adopted for this addition.
The proof assays should contain approximately the same proportion of nickel as the sample.
9.2.2 Scorification
For white gold alloys containing nickel, pretreatment of the sample by scorification involves wrapping it in 2 g of lead foil. The sample consists of 125 to 250 mg of gold and is inquarted with silver equivalent to 2.3 to 3 times the mass of fine gold present. Place this capsule in a scorification dish, together with 15 g of lead and 1.5 to 2 g of anhydrous borax and heat to 1000°C in the furnace. An increased air supply may be needed to oxidize the large quantity of lead. After 20 to 30 min, when a liquid slag covers the surface of the dish, raise the temperature briefly to I 100°C (for about 2 min). Remove the dish with the tongs, cool and separate the lead button from the slag. This button, which contains the original gold and silver, is cupelled as described in 9.1.3.
9.3 White Gold Alloys Containing Palladium (Nickel Absent)
For white gold alloys containing palladium, traces of this metal may remain in the cornet after a single cupellation and parting. With these alloys, the cornet from the samples and the proof assays should be recupelled with 4 g of lead, silver equal to 2.5 times the mass of gold and a small piece (about 50 mg) of copper. Repeat the parting process and weigh the final cornets.
The proof assay should contain approximately the same amount of palladium at the sample.
9.4 Gold Alloys Incorporating More than 40 Percent Silver
These alloys shall be treated as yellow gold alloys, with proper allowance being made for the higher silver content when determining the inquartation addition.
9.5 Alloys Containing 990 (%.) and Above Gold
When analyzing samples containing approximately 990 (%o) gold, still increased accuracy in operation and parameter control is needed.
In order to achieve the best results, proceed as stated in 9.1, introducing the following modifications:
a) Weigh at least 250 mg of alloy; add 20 ± 5 mg of copper to the sample and amount of inquartation silver as stated in 8.2.1;
b) For the proof assay samples, proceed exactly in the same way as for the assay sample. Use gold of purity 999.9 (%o) and take care the mass of the added inquartation silver is in the same range ± 10 mg) as for the assay samples. Always run in parallel at least two proof assay samples:
c) Carry out the cupellation with a total amount of at least 2 g of lead;
d) After cupellation, flatten all beads so that they have approximately the same shape and thickness; anneal the flattened beads in a muffle to red heat (700°C) to obtain the same condition of recrystallization; and
e) Proceed to the parting of the samples as stated in 9.1.4. Take care that the quantity of acid and the parting time are the same for all samples of the same series. Finally, dry and anneal in parallel all fine gold cornets. The use of a basket for the parting will be advantageous for this purpose.
NOTE — The cupellation will take about 15 min
Method of Calculation
Calculate the gold content, W in parts per thousand (%o) by mass, of the alloy using the formula:
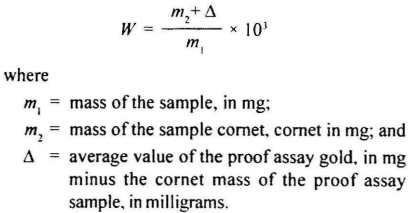
Assay Repeatability
Duplicate determination shall give results differing by maximum 0.5 parts per thousand (%o) by mass for yellow and red gold alloys, maximum 1.0 part per thousand (%o) by mass for white gold alloys and maximum 0.2 parts per thousand (%o) by mass for gold alloys containing 990 (%o) or more gold. If the difference is greater than this, the assay shall be repeated.
When analyzing alloys with a fine gold content of 990 (%o) or more, the values of for the proof samples (see 9.1) which run in parallel shall not differ by more than 0.04 mg. If the difference is greater than this, the assay shall be repeated.
GOLD ASSAY REPORT
The test report shall include the following information:
a) Identification of the sample including source, date of receipt, form of sample;
b) Sampling procedure;
c) The method used by reference to this standard;
d) Gold content of the sample, in parts per thousand (0/00) by mass, as single and mean values;
e) If relevant any deviations from the method specified in this standard;
f) Any unusual features observed during the determination;
g) Date of test;
h) Identification of the laboratory carrying out the analysis; and
i) Signature of laboratory manager and operator.
