The density of a Flocculant is usually evaluated by measuring its buoyant density while settling in a quiescent column. An equation of the general form (Matsumoto and Mori, 1975)
![]()
is used to calculate the buoyant density (pa), from settling rate (U) and diameter of floc (Df). The constants Kf, α, β and γ depend on shape, porosity and the flow conditions [viscous or turbulent] around the floc. For a rigid sphere falling under viscous flow conditions equation (1) reduces to
U = 0.056 g Pa Df²/µ……………………………………………………………………………..(2)
It is known that shape and porosity of flocs vary widely and it is thus difficult to establish a meaningful correlation between the constants in equation (1) with the above factors. The situation is further complicated by the fact that porosity of a floc varies with floc size.
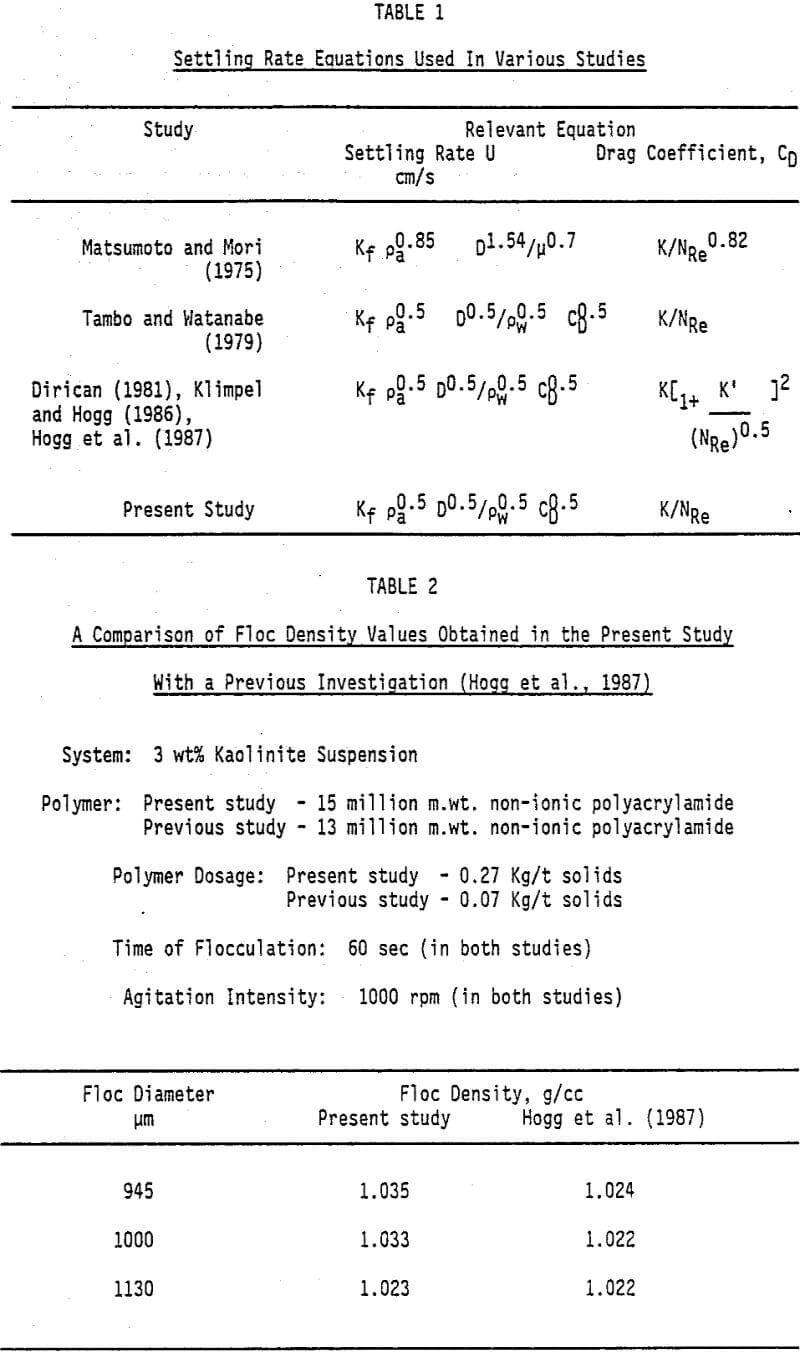
Various investigations, carried out to evaluate floc densities, have been reported in the literature. In all these studies a in situ photographic technique was used to measure the size of a falling floc and diameter was calculated from the projected area. However, the equation used to calculate the floc densities were different and none of the studies accounted for shape and porosity of the floc in an appropriate manner. The different settling rate equations used by various investigators are summarized in Table 1. It appears that in the past studies the equation to represent the settling rate of the flocs was selected on the basis of least statistical error obtained in evaluation of the power law relationship between floc diameter and its density.
In the present study, floc density measurements were conducted using a simplified procedure than employed by previous investigators. The experimental technique involved transfer of flocculated mass from the mixing vessel into a large tray, to prevent reagglomeration of flocs, followed by transfer of individual floc onto a glass slide. The slide was placed under a microscope, provided with a grid, and the projected area was measured. The floc was then gently introduced into a settling column and terminal velocity was measured. In order to establish the reliability of our procedure, a comparison of floc density obtained, for a kaolinite suspension flocculated with a non-ionic polyacrylamide, was made with the values reported in a previous study. The results presented in Table 2 showed a good agreement between the densities obtained in the two studies.
It has been shown that floc densities can be measured by using a relatively simple procedure. The effect of different variables on floc density indicated that chemical factors such as chemical nature and dosage of the flocculant play a more significant role than physical parameters. For example, pulp density and agitation intensity did not have any effect on resulting floc density while significant variation in density was observed with a change in the chemical nature of the flocculant used. The floc density was also found to vary inversely with floc size. The effect of variables on floc density is explained on the basis of aggregation of particles during flocculation rather than floc density distribution obtained after disintegration of flocs.