Table of Contents
Lime is a very general term applied to products of limestone, in popular treatises often incorrectly, including ground or pulverized limestone used in agriculture. When used without qualifying adjective, the term usually means burned or calcined limestone, or quicklime, or calcia.
Limestone is burned (roasted or calcined) in furnaces known as lime kilns by direct exposure to the hot gases and flames. When heated to incandescence, the carbon dioxide content of the rock is expelled, leaving calcium and magnesium oxides, according to the formula usually written:
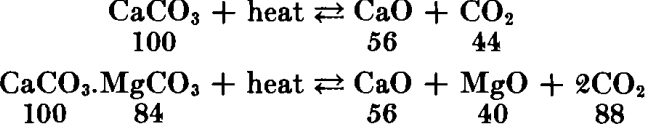
Lime has great affinity for moisture and carbon dioxide; therefore it is a perishable commodity. When combination occurs slowly, due to exposure to the atmosphere, the resulting product is “air-slaked.” Since the reaction with water involves the evolution of considerable heat, lime cannot safely be shipped or stored in bulk unless amply protected. Fires in railway cars and storehouses have been caused by leaking rain water. Consequently, much of the commercial lime is prepared in the form of a dry hydrate powder, which is a more stable and readily handled commodity. Since nearly all industrial uses of lime involve association with water, hydrated lime (calcium hydroxide) is interchangeable with quicklime for practically all purposes. The hydrating is done mechanically with just the right amount of water, according to the chemical reaction:
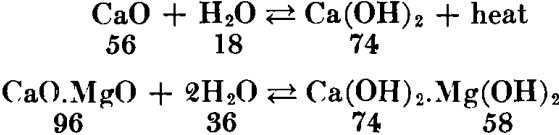
Properties
Lime owes its industrial value primarily to the fact that it is the commonest and cheapest of alkali chemicals; therefore it is widely used as a base, causticizer, and neutralizer. For construction purposes, lime is the cheapest mortar and plaster material, primarily because of its physical properties for malting plastic mixtures, in relatively lean proportions, that harden on exposure to moist air. In agriculture, its value is threefold: (1) it neutralizes acid soils; (2) it improves the physical condition of heavy soils; (3) it supplies calcium, magnesium, and some of the rarer mineral food requirements of growing plants and animals.
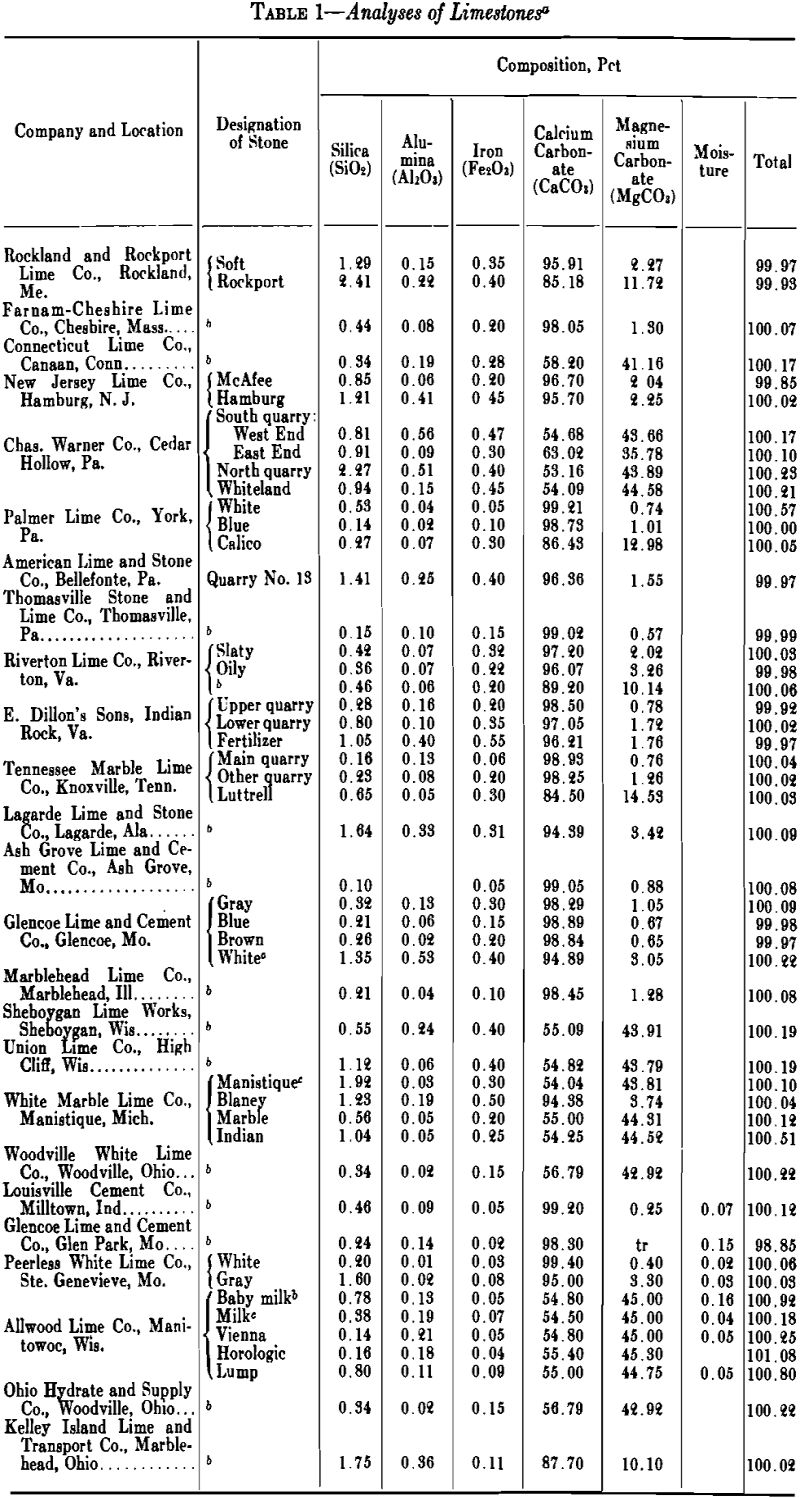
The value of lime for most purposes is dependent on its content of available calcium oxide, or the sum of its available calcium and magnesium oxides, for the particular process involved. A few limestones contain more than 99 pct pure carbonates but the majority of limestones are seldom more than 97 pct pure. The chief impurities are silica, alumina, and iron, which react with the calcium oxide in the kilns and tie up a part of the lime in mineral forms unavailable for chemical uses but are not detrimental to its use in construction. Where the percentages of silica and alumina are considerable, and the silica is present as a silicate rather than quartz, the product made is hydraulic lime, which may be preferred for mortar, or even for plaster, since it has considerable tensile and compressive strength when hardened.
Limestone-Origin and Mode of Occurrence
Limestone (or lime rock) is the source of raw material for most of the lime manufactured but calcium carbonate or hydrate from any source may be used in a properly selected type of kiln. Crushed and washed oyster shells are used to a considerable extent; also calcium carbonate and hydrate wastes from the paper and other industries. Such waste, in the form of sludge or slurry, is calcined in rotary kilns, usually with the addition of limestone.
Limestones are sedimentary rocks, which occur in practically inexhaustible quantity in nearly all parts of the world and in an infinite number of varieties. The calcium and magnesium carbonate minerals of which limestones are largely composed are calcite, aragonite, dolomite and magnesite. According to F. W. Clarke (The Data of Geochemistry, U. S. Geol. Survey, Bull. 695, 4th Ed., 1920), these four minerals have the following characteristics:
Calcite-Rhombohedral. Composition, CaCO3. Molecular weight, 100.1. Specific gravity, 2.72. Molecular volume, 36.8. Hardness, 3. Normally, colorless, but often variously colored by impurities.
Aragonite-Orthorhombic. Composition, CaCO3, like calcite. Specific gravity, 2.94. Molecular volume, 34. Hardness, 3.5 to 4. Color, white, but often tinted by impurities.
Prospecting and Exploration
The extent to which prospecting and exploration of limestone deposits should be carried out depends upon several factors: (1) the size of the expected operation, (2) the extent and character of the exposed ledge, (3) the kind of lime product to be made, (4) the kind of mining operation to be employed, and others. Many small limestone quarries have been opened and worked for years. Mere sampling of the exposed face at various places may be all that is required for these or a moderate-sized operation, and hundreds of small lime plants with two or more shaft kilns have been established on such data. On the other hand, the number of these small operations in the lime industry has been steadily diminishing for many years, and the lime industry in the United States in 1945 had become one of only 189 producers, compared with more than twice that number in the 1920s and about five times that number in the early years of the present century. Of the 189 active producers, 73, producing annually from 25,000 to 100,000 tons or more each, accounted for 87 pct of the entire tonnage. It follows, therefore, that a modern lime plant, requiring a capital investment of probably not less than half a million dollars, justifies a scientific determination of the quality and extent of the limestone deposit.
The rapid decrease in the number of lime-plant operations and the increase in size of the survivors have been due not only to the stronger commercial position of the large producers but to the fact that they have introduced scientific methods of exploring and developing their
Since core-drilling is common to all mineral industries, it is not considered necessary here to explain in detail the methods employed in exploring a limestone deposit. Such a survey is simpler than locating the vein of an ore body because often all that is necessary is to determine the depth of overburden and the boundaries of the deposit and to find out whether or not it is of fairly constant composition.
Mining and Quarrying Methods
By far the largest tonnage of limestone used for lime manufacture is obtained by open quarrying, because in many important lime-producing areas the limestone deposits are in horizontal or only slightly dipping strata, usually accessible without excessive stripping operations. Open-pit methods have been widely adopted because both producers and labor available are more familiar with quarrying than with underground mining, and because in most large operations considerable volumes of limestone are produced for other products than lime.
Where the operation is exclusively for the production of lime in vertical or shaft kilns, the quarrying is very simple. The object is to avoid production of fines, and drilling and blasting are designed accordingly. The use of compressed-air drills, both the hand-hammer type and mounted wagon drills, is regular practice. The rock is often hand-picked and loaded into small industrial railway cars or into skips, which are picked up by specially designed motor trucks or by cableways. The cars or skips are then conveyed direct to the tops of the kilns and dumped. Such an operation requires a minimum of mechanical equipment and in isolated localities and for relatively small production is still normal, good practice.
It was common practice in the manufacture of dolomitic finishing lime in Ohio, to use steam injectors under the grates or directly into the fireboxes, in order to prevent overburning of the magnesia component of the lime. Recent installations have substituted CO2 for steam, with satisfactory results.
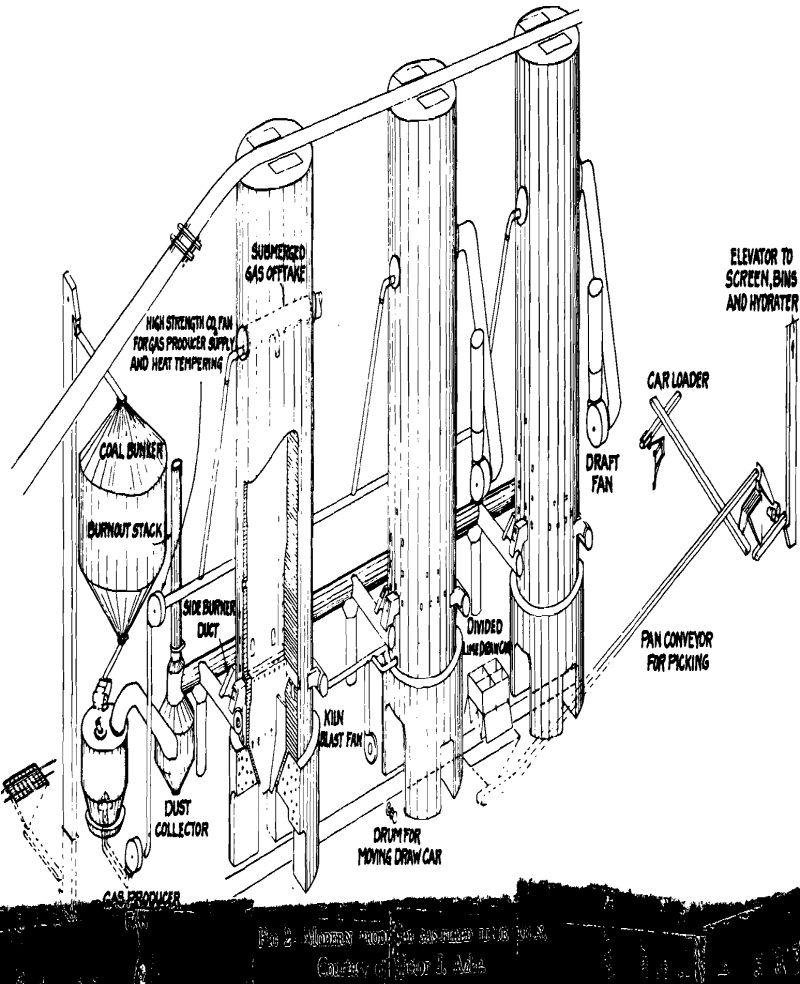
Efficient combustion and utilization of the heat units in the calcining or burning zone of the kiln is only one phase of the problem, however. Limestone does not begin to evolve CO2 gas freely until a temperature of approximately 1800°F is reached under normal kiln conditions. On laboratory samples, the temperature of dissociation of calcium carbonate is about 1650°F and that of magnesium carbonate about 1400°F. The actual temperatures under operating conditions are never less than 2000°, usually between 2000° and 2500°, in order to avoid long periods that would be required to completely calcine the limestone at lower temperatures. The actual time required for a piece of rock (later lime) to travel through a shaft kiln into the lime-cooling zone has been found to vary widely in different kilns, from less than 2 days to 8 days, depending on the method of firing the kiln as well as on the kind of lime or limestone. The shorter periods are for gas-fired kilns.
Operation of Kilns
Operation of shaft lime kilns follows two general practices depending on, the design of the kiln, the characteristics of the limestone and of the kiln lining, and the operator’s experience. Originally the shafts of kilns were walls of limestone but as these suffered from partial calcination and abrasion it became the practice to line them with refractory brick or block. Since nearly all refractories in common use are acidic in reaction and lime is basic, there is a tendency for the hot lime to react with the lining and to stick to it. This, and the arching effect of pieces of rock in a shaft, makes a kiln “hang.” This tendency is made use of in operating many kilns to draw the finished lime from the cooler and to drop the lime in the calcining zone into the cooler. The partly burned stone and limestone above where the kiln is hung is then poked down with iron bars through poke holes and firebox. The drawing and poking are done at intervals a few hours apart, when fuel supply is cut off.
Calcination of limestone in rotary kilns probably accounts for more than half the total tonnage of lime produced in the United States at the present time. They became popular as markets developed for granulated and pulverized quicklime in the chemical industry. The use of rotaries makes possible utilization of crushed stone to about ¼-in. size and parhard-burned or sintered dolomite, classified as “refractory lime.” The preparation of the dolomite for calcining includes crushing, usually with rolls, to reduce the amount of fines, and mixing with the sized dolomite a small percentage of iron ore, boiler scale, or other iron ingredient. The mixture is then calcined at sintering temperatures of about 2800°F. The product is granular and is shipped chiefly in bulk in boxcars, direct to the steel plants, for lining the bottoms of open-hearth furnaces. It is used also for the manufacture of dolomite refractory brick.
As a lime kiln, the reactor is a steel cylinder lined with refractory and placed in an upright position. In other words, it resembles an ordinary shaft (vertical) lime kiln. It is divided by perforated, domed, refractory constriction plates into three or more compartments, as shown in Fig 6. These compartments correspond to the preheating, calcining, and cooling zones of an ordinary shaft lime kiln.
The warm air then passes through the calcining compartment, where the fuel is injected, and the products of combustion then pass to upper compartments to preheat the limestone. As it progresses upward, the gas pressure diminishes so that in the preheating, or initial fluidizing,
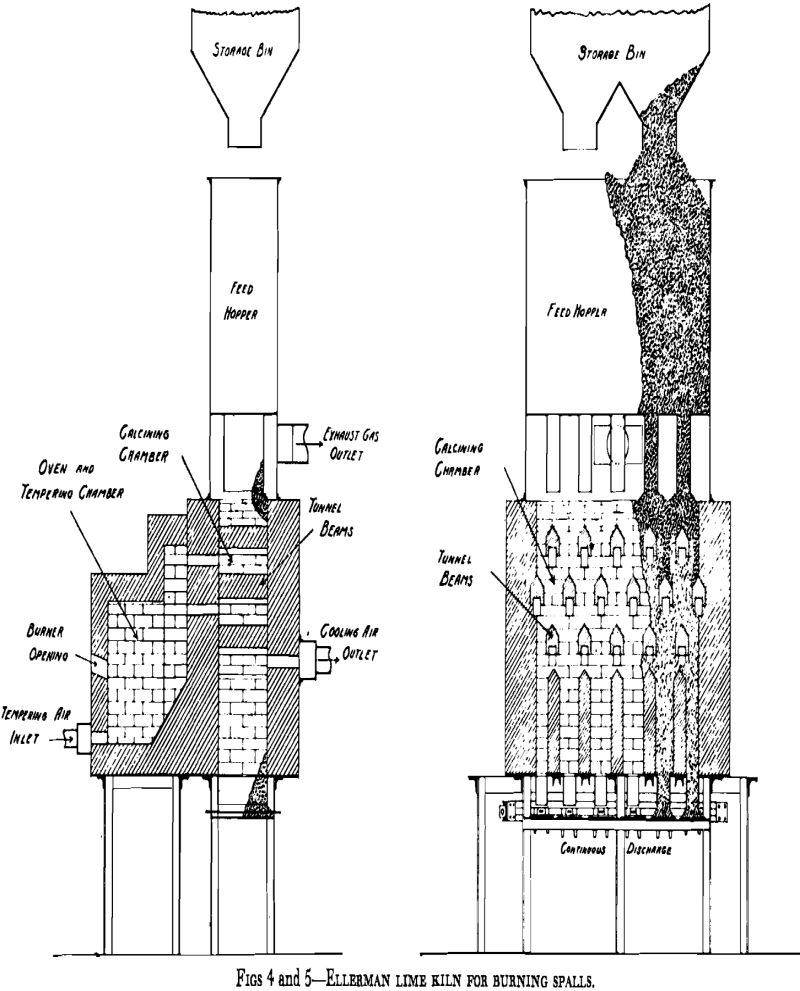
compartment, the pressure normally is less than one pound per square inch.
Handling the Product
The calcined product of any kind of lime kiln may require careful selection or hand picking in preparation for the markets, to avoid “core” or pieces of uncalcined rock. This is particularly true of the product of shaft lime kilns, for which it is common practice to spread the drawn lime on the floor of the shipping room or shed and inspect all large or suspicious pieces by breaking them up with a hammer. A more modern method is to inspect and hand-sort the lumps as they pass between two workmen on opposite sides of a picking conveyor.
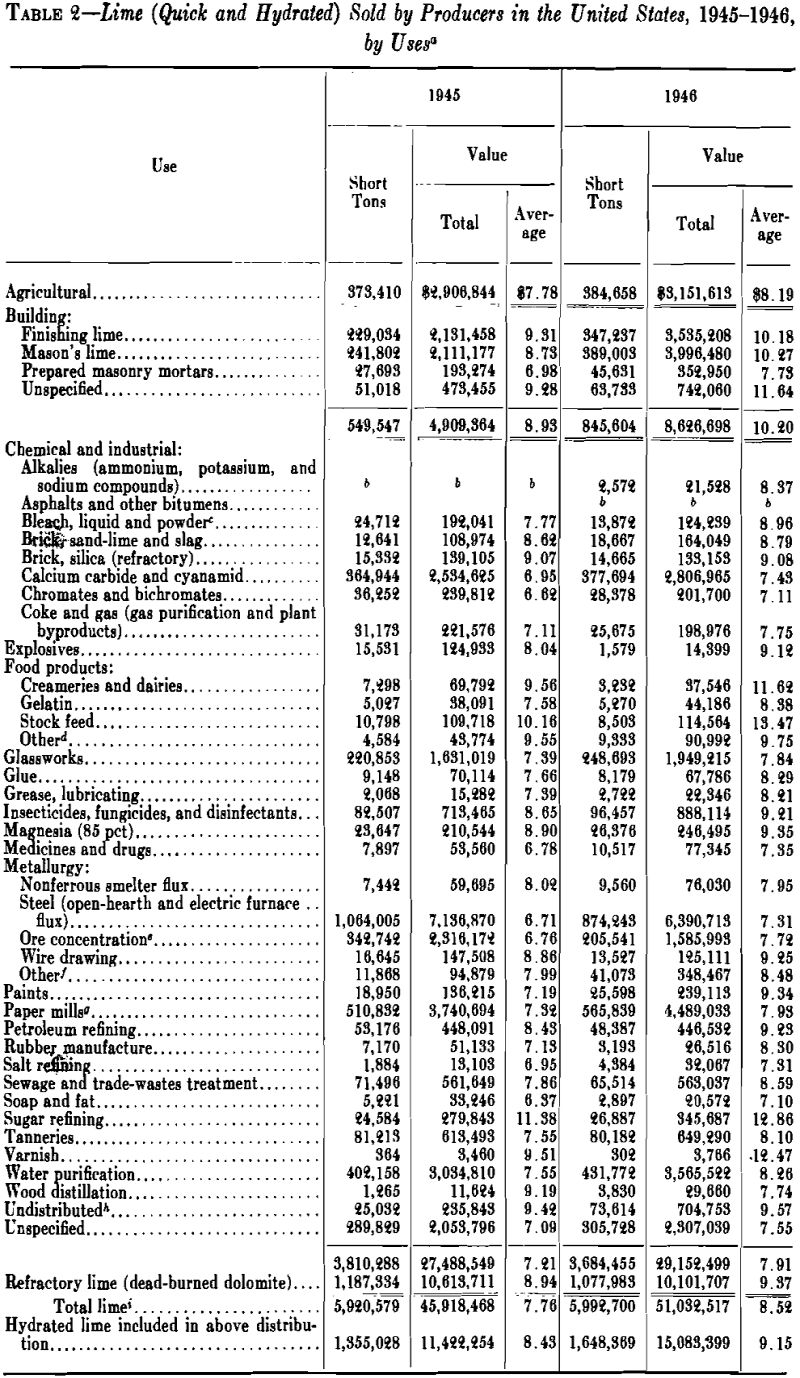
Lime is prepared for the market in the following forms: (1) large lump, 8 in. and smaller; (2) pebble or crushed, 2½ in. and smaller; (3) ground, screened or granular, ¼ in. and smaller; (4) pulverized, substantially all passing a No. 20 (840-micron) screen; (5) standard hydrated lime; (6) superfine hydrated lime. The first requires no preparation except packing in barrels or similar containers; the second, pebble lime, is usually a lime that naturally disintegrates to pebble or granular size in the kiln; crushed lime is lump crushed to pebble or granular size; pulverized lime is lump or granular lime reduced to powder, usually in a hammer or ring-roll mill, with screen or air separation. Pulverized quicklime and hydrate are difficult to screen through fine meshes because they are repulsed from steel wire by an electrostatic charge, which builds up on the particles.
Since the value of lime in mixtures of portland cement, lime and sand is based largely on the capacity of the lime for retention of water, thus providing plasticity and workability and proper moisture conditions for hardening and curing, various tentative tests have been proposed to determine and compare this physical property of hydrated limes. According to the present tentative method of the ASTM, a special piece of equipment is required, which measures the flowability of the mixture after the amount of water that can be sucked out of a mortar specimen in one minute is removed. Other methods suggested include an extrusion device for testing the plasticity or flowability of the mortar.
