Estimation of Copper in Ores by Iodide Method
The estimation of Copper in Ores by the Iodide method.—The standard solution is again used, and needs no further checking. The student may take for
The estimation of Copper in Ores by the Iodide method.—The standard solution is again used, and needs no further checking. The student may take for
Apparatus and Reagents.—The usual volumetric and gravimetric apparatus. The student may take for analysis a sample of the ordinary bench reagent 5E. HCl. He will
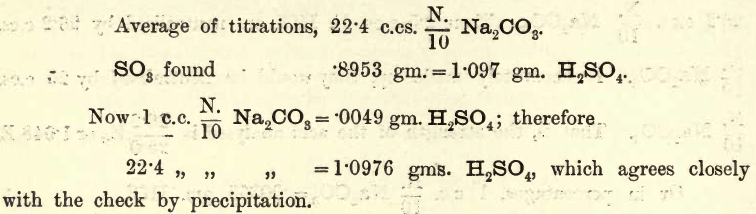
Apparatus, Reagents,—The usual volumetric and gravimetric apparatus. For analysis the student may take the bench reagent labelled 5E. NH4HO. For the standard acid pure H2SO4

Assuming that assaying be defined as “the estimation of the commercially important elements in their ores, alloys, or products,” the following classification of methods, though
Method of Experiment.—A number of charges of a quartzose ore are prepared, and weighed quantities of certain substances are added to the various charges. The
Method of Experiment.—Weighed quantities of other metals will be added to weighed quantities of silver and lead, and after cupellation the coloration of the cupel

The Iron or Nail Method Principle: Sufficient soda is added to flux the silica, and an excess to give a fluid alkaline slag in which
This material may be taken as containing 96 to 98% copper, a few ounces of gold per ton (varies considerably with different ores), some hundreds

To estimate how much silver is present in galena, the student has already assayed this ore for lead, and by cupelling the buttons so obtained
The following estimation is given as an exercise in distillation. The method has been used to advantage, being convenient and requiring little apparatus. For refined