Table of Contents
As part of a continuing effort to maximize metal recovery from domestic secondary resources, the Bureau of Mines investigated a process for recovering the metallic portion of scrap alkaline batteries. A pyrometallurgical method for recovering nickel and cadmium from Ni-Cd scrap batteries previously developed on a laboratory scale, was scaled up to 25- and 43-pound charges. The method employed reduction and/or decomposition in a retort using a minimum of 2.5 percent carbon as a reductant. Metallic cadmium was distilled at atmospheric pressure and a minimum of 900° C. Purity of the recovered cadmium was 99.8 percent minimum and the nickel-iron residue contained less than 0.02 percent cadmium.
The manufacture and use of nickel-cadmium alkaline batteries came into relative prominence in the late 1950’s. During the period of 1966-71, approximately 3 percent of the United States primary cadmium demand was consumed by battery manufacturers. From 1971 to 1975, the demand grew from 3 to 13 percent. It was estimated by the battery manufacturers that by 1981, 2.2 million pounds or greater than 20 percent of the United States demand for cadmium will be consumed by the battery industry. The United States is dependent on Canada, Mexico, and Australia for more than 60 percent of its primary cadmium supply.
Virtually all of the Ni-Cd battery scrap reportedly is being exported overseas where it is processed and returned to this country as refined metals. Several scrap dealers are breaking the battery cells and hand separating the positive and negative plates. The positive plates, containing 1 to 2 percent cadmium, are smelted in the United States to a high ferronickel alloy or used in producing high-nickel alloys. The cadmium oxide fume from this smelting operation must be controlled to meet EPA emission standards. The cadmium-rich negative plates are currently exported overseas.
Prior to this investigation, only the following three methods were known for processing scrap Ni-Cd batteries: A hydrometallurgical method developed by the Bureau of Mines in 1971, a sulfuric acid-leach and electrolytic method, and a pyrometallurgical method patented in France for which no details were available. Bureau of Mines research has led to the development of a pyrometallurgical method for recovering metallic cadmium and a nickel-iron residue low in cadmium. A maximum concentration of 0.1 percent cadmium is permitted for effective recycling of the nickel-iron residue.
Battery Construction and Composition
Nickel-cadmium batteries consist of the following essential parts: grids, paste, active materials, separators, electrolyte, and cell cases. The grid material is a wire mesh cloth or perforated metal sheet. The wire cloth is usually pure nickel, but nickel-plated iron has been used also. When perforated metal sheet is used, the base material is plated with nickel. Nickel powder is applied to the grid material as paste and sintered at 800° to 1,000° C. The result is a porous material that will absorb the active materials to form positive and negative electrodes (plates). The positive plates are impregnated with an Ni(NO3)2 solution and the negative plates with a Cd(NO3)2 solution. Roasting and immersion in a caustic solution produces active materials of Ni(OH)2 and Cd(OH)2, which are polarized to produce a charged or active electrode. The impregnation cycle is repeated four to five times yielding a weight gain of 1.1 gram Ni(OH)2/cm³ and 1.6 gram Cd(OH)2/cm³ of plate volume, respectively. Normally, sintered type cells are filled with an electrolyte of pure KOH with a density of 1.25 to 1.30 g/ml. Some manufacturers, however, will also add LiOH (15 to 30 g/l) to the electrolyte to improve the capacity of the positive plates. Thus, the material normally found in battery plates are Ni, Ni(OH)2, Cd, Cd(OH)2, and Ni or Fe grid material. The main reaction (2) of the Ni-Cd cell on discharge and charge is:
2βNiOOH + Cd° + 2H2O ↔ 2Ni(OH)2 + Cd(OH)2………………………………..(1)
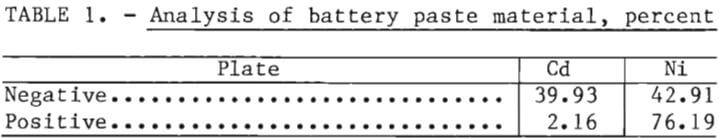
On exposure to the atmosphere the battery plates and KOH electrolyte absorb CO2 to form a basic nickel carbonate [NiCO3·NiO], CdCO3, and K2CO3 or KHCO3. Several battery cells were completely dismantled and random samples were taken from positive and negative plates and analyzed for cadmium and nickel content. Analysis of the battery paste material recovered from these plates is shown in table 1.
When the plates are assembled, a separator is used between each positive and negative plate. Most of the separator materials are plastics, usually in the form of thin porous sheets. Nylon and polypropylene are the two most common plastic separators used today. Plastic is also the most common battery case material for open sintered cells. Polystyrene is used for all commercial batteries and nylon for certain military batteries.
Bench-Scale Tests
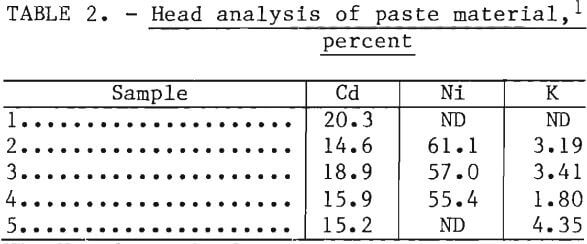
Battery paste was used for small-scale testing (150-250 grams). The paste was removed from the plates either by ball-milling or shredding in a hammer mill with ½-in diam grates. The ball-milled material was screened on 80 mesh. Paste material from the hammer mill was screened through 10 mesh. Magnetic materials, which consisted of iron-base plates, were removed with a hand magnet. Typical head analyses are shown in table 2.
Various conditions were studied during bench-scale testing. These included the effects of carbon, pressure, and temperature on the distillation and recovery of cadmium. Results from these tests showed that cadmium could be effectively distilled from the battery scrap charge at atmospheric pressure. About 99.8 percent of the cadmium was recovered as a high-purity condensate. Optimum results were obtained at 900° C for 2 hours using 2.5 percent carbon in the charge. Coconut charcoal was the source of carbon for all tests. Details of these tests have been published previously.
Explosion of Cadmium Condensates
Bench-scale tests revealed that cadmium condensates could become explosive if the charge is reduced and distilled at a low vacuum of 400 microns and 950° C. The exact cause was never identified but the cadmium condensates that exploded or sparked contained greater than 10 percent potassium whereas the nonexplosive condensates contained less than 2 percent potassium. Since effective reduction and distillation can be accomplished at atmospheric pressure, and in view of the potential explosion hazards with vacuum distillation, the latter is not recommended.
Larger Scale Tests
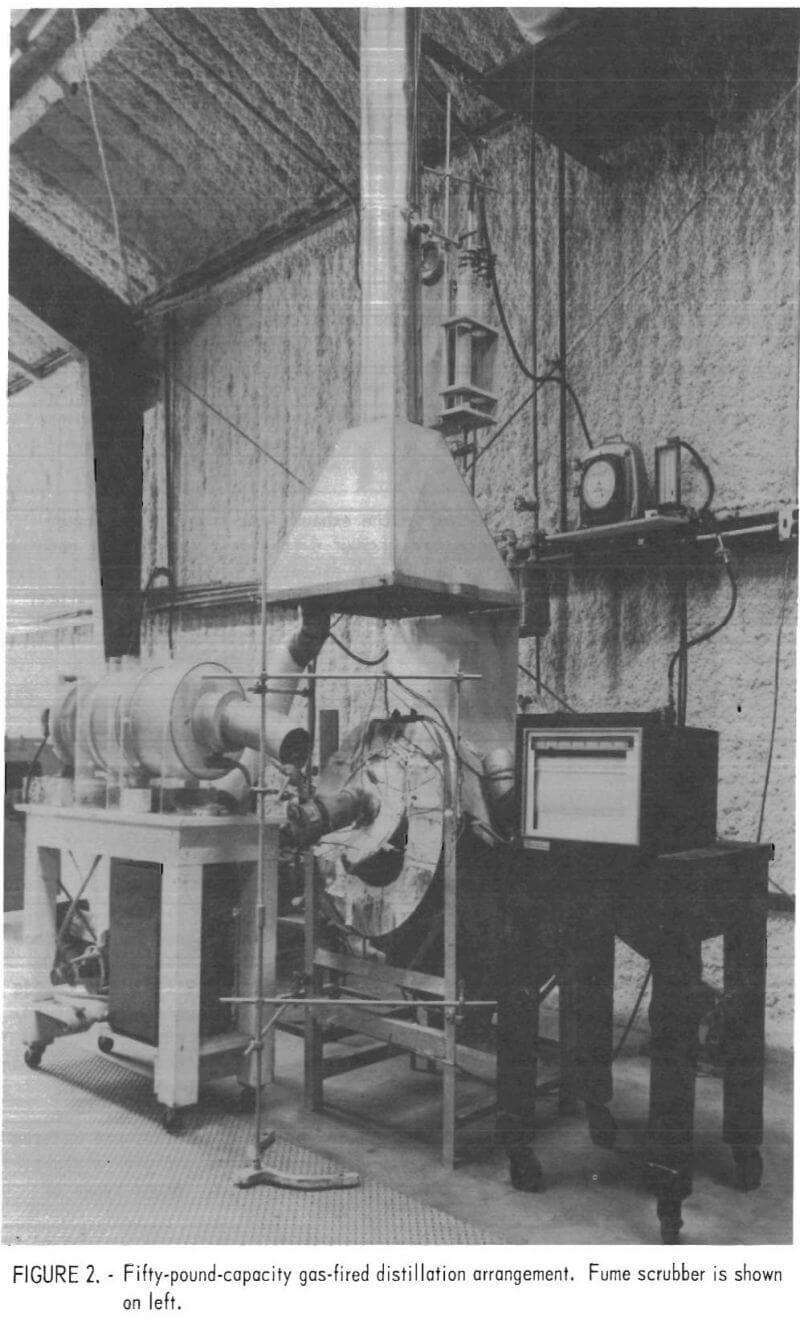
Larger scale tests were conducted in a gas-fired retort. Depending on the size of the crucible used as the retort, charge capacity ranged up to 50 pounds. The general construction and arrangement of the gas-fired retort
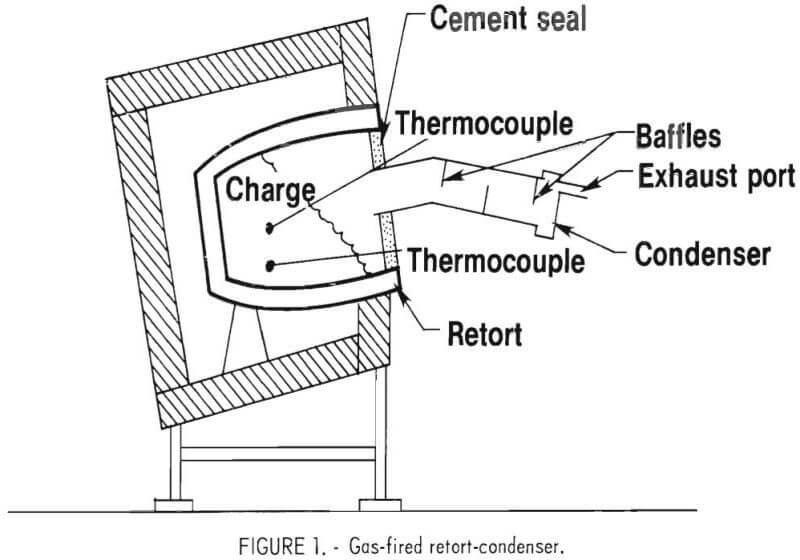
and condenser are shown in figure 1. Evaluations made with this retort included: Retort materials, condenser design, emissions, product analysis, residue fusion, and heat distribution. A number of modifications were required during these tests, such as baffle relocation in the condenser, condenser insulation, and variation of water and emission collection systems. The final furnace design involved a 50-pound clay-graphite retort. This furnace and condenser arrangement is shown in figure 2. A wet scrubber used for collecting the condenser emissions is shown to the left of the retort.
Retort Material
Two types of material were tested for suitability as retort refractory for the pyrometallurgical process, Refrax 20, and clay graphite. Refrax 20, a silicon-nitride-bonded silicon carbide, was originally recommended because there are no known reactions with the battery and flux charge components at temperatures up to 2,200° F (1,204° C) in a reducing atmosphere. During initial tests, however, this material exhibited excessive porosity permitting cadmium vapor to escape through the retort. Clay-graphite crucibles were subsequently used for the retorts and gave excellent service, although fusion of the battery scrap to the glazed retort surface occurred at temperatures greater than 1,000° C. Light fusion of the scrap to the retort surface also occurred at temperatures of 900° to 1,000° C.
Cadmium Condenser Design
The condenser (fig. 1) consisted of a 3-in-diam iron pipe equipped with a screw cap on the end to facilitate removal of metallic cadmium condensate. A plug was later added so that molten cadmium could be tapped from the condenser. Baffles were placed in the condenser at numerous locations to minimize cadmium emissions from the exhaust port. The most effective arrangement appeared to be four sheet metal baffles at the back of the condenser spaced ½-inch apart. Insulation was used on the outside of the condenser to permit condensing of the cadmium as liquid for easier recovery by tapping directly from the condenser. Tests were run with and without insulation to determine the optimum temperature levels within the condenser. With insulation around the outside of the condenser, temperatures at the retort end rose to almost 600° C and the cool end was 300° to 500° C. With the insulation removed, maximum temperature at the retort end was 500° C and at the cool end, 270° C. These tests revealed that operation with the external insulation removed was preferred, particularly since this arrangement minimized cadmium emissions. At the completion of each test, external heat was applied to the condenser to melt and tap the cadmium condensate.
Cadmium emissions were detected in the exhaust gas from the condenser when the retort had reached approximately 500° C (the melting point of cadmium is 321° C). The emissions are caused by the evolution of water vapor and carbon monoxide which act as carrier gases. Water and carbon monoxide are produced by the following reactions in an approximate temperature range of 100° to 1,000° C:
Cd(OH) 2 → CdO + H2O…………………………………………………(2)
Ni(OH)2 → NiO + H2O…………………………………………………..(3)
CdCO3 → CdO + CO2……………………………………………………..(4)
2CdO → 2 Cd + O2…………………………………………………………(5)
CdO + C → Cd + CO……………………………………………………….(6)
2C + O2 → 2CO…………………………………………………………….(7)
Water was also contributed by the retort charge which contained some absorbed moisture and was not deliberately dried prior to testing. Based upon a 10- pound charge containing 15 percent cadmium, a maximum of 119 liters of CO gas would be produced according to equations 5, 6, and 7. Attempts were made to condense the water vapor and cadmium dust using a condenser and/or a packed column. Blockages occurred causing back pressure in the retort-condenser system which resulted in cadmium oxide (CdO) leaks. An average of 480 ml of water was condensed and 1.8 to 22.8 grams of cadmium were collected. The problem of collecting the mixture of water vapor, carbon dioxide, and cadmium dust was alleviated by using a wet scrubber (Mystain) equipped with a plastic mesh filter screen for removing the wet cadmium particles. Other types of filters or scrubbers are available that could be used.
Battery Material Preparation
Test material for all the large-scale tests was prepared by first breaking the plastic battery cases, then removing the plates and allowing them to drain and partially air dry. Very little KOH electrolyte drained from the battery case or plates. The nylon separators were removed and the plates were cut or torn from the battery connecting posts. The plates were shredded in a hammer mill with ½-in-diam grate openings. Observations during shredding revealed that an optimum amount of moisture was required to avoid dust problems, without causing adverse caking inside the mill. A moisture content of 5 to 10 percent was judged adequate to meet these requirements. After shredding, approximately 80 to 85 percent of the material passed a 10-mesh screen. The minus 10-mesh material was almost all paste material with approximately 5 to 10 percent grid material, whereas the plus 10-mesh material was mostly grid material (wire and steel punch plate) with a small amount of adhering battery paste.
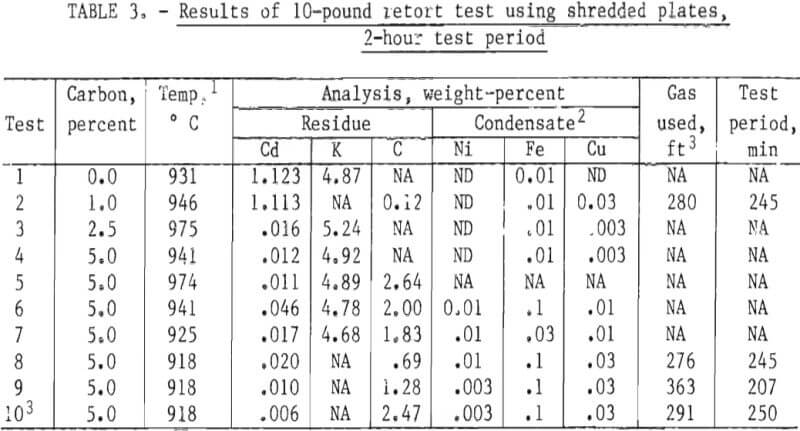
Ten-Pound Retort Tests
A number 40-size clay-graphite crucible with a capacity of 10 to 15 pounds of charge was used in the gas-fired retort for the 10-pound tests. All tests were run at 900° C or higher for 2 hours with 10 pounds of material. Coconut charcoal was the source of carbon for all tests. All tests used shredded plate material except test 10 which used whole plates. Results are tabulated in table 3. All tests with 2.5 or 5.0 percent carbon resulted in only 0.006 to 0.046 percent cadmium remaining in the residue. All cadmium condensates were analyzed spectrographically. Nickel, iron, and copper were the only impurities detected and thus by difference showed a minimum purity of 99.8 percent. The source of the copper is unknown, but is believed to be associated with the plating of nickel and iron grids used to make some of the battery plates.
Test 10 resulted in the lowest residual cadmium, 0.006 percent. The main difference between this and the preceding tests was the use of whole plates instead of shredded plates. Unreacted carbon was relatively high (2.7 percent of the residue), and the degree of fusion in the residue appeared very high compared to the other tests. Fusion is of possible commercial concern if it is extensive enough to interfere with removal of the residue for further processing. The bulk density of the unshredded plates was appreciably lower than the shredded plates causing difficulty in charging the total 10 pounds of charge into the retort. Bulk densities were not measured directly but rough estimates indicate 55 to 65 lb/ft³ and 40 to 50 lb/ft³ for shredded and unshredded plates, respectively. Estimates were based on judging the approximate volume occupied in the full retort after a given weight of plates were chosen. For relative information purposes, a gas meter was used in tests 2, 8, 9, and 10 to determine fuel used during a complete test. The gas used (shown in table 3) includes the heat-up period to reach test temperature.
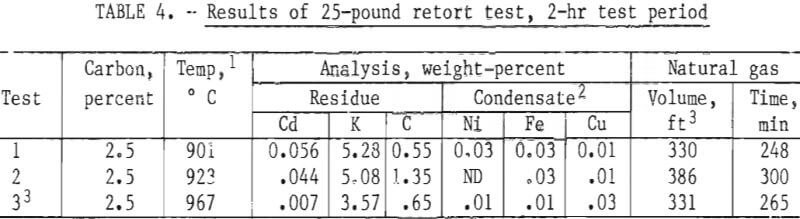
Twenty-Five-Pound Retort Tests
The next series of tests were conducted with nominal 25-pound charges. The retort was fitted with a number 100-size clay-graphite crucible having a charge capacity up to 50 pounds. Temperature was measured in front, center, and rear portions of the charge. The 2-hour holding period was initiated when the average of the temperatures reached 900° C or higher. The average temperature variation between the coldest and the hottest regions of the charge was 245° C. Test conditions and results are shown in table 4. Cadmium in the residue varied from 0.007 and 0.056 percent and the cadmium condensate purity was approximately 99.9 percent. The carbon addition for the three 25-pound tests was 2.5 percent.
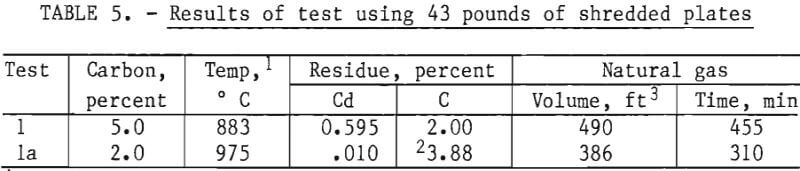
Forty-Three-Pound Retort Tests
Scale of processing was further increased in a final test using 43 pounds of shredded plates. To insure sufficient amount of carbon for reduction, 5 percent carbon was used. The heat-up time to reach test temperature was approximately 5½ hours, compared to 1½ to 2, and 2 to 3 hours heat-up time for the 10- and 25-pound tests, respectively. Heat distribution was poor during this test as revealed by the wide temperature variation for the three thermocouple locations, approximate front, middle, and rear of the charge. During the test period, the three locations average 883° C, but ranged from 800° C at the front to a maximum of 1,121° C at the rear of the charge. The test was initially terminated after a 2-hour holding period at temperature. As shown in table 5 (test 1), the residue still contained 0.595 percent cadmium. After analysis, this residue was recharged to the retort without additional carbon. The holding period was not initiated until the average temperature of the three locations was 900° C minimum. The average for the 2-hour test (holding period,) was 975° C. The increased temperature caused appreciable fusion of some of the charge to the clay-graphite crucible. Some of the crucible was thus removed with the residue resulting in a higher carbon analysis than at the start of this second test period (test 1a). Analysis of the residue after completion of test 1a showed that the cadmium concentration was reduced to 0.010 percent, well below the 0.10 percent reportedly required to permit effective recycling. The cadmium condensate, shown in table 6, was high purity, approximately 99.8 percent with only minor concentrations of Ni, Fe, Ca, Pb, and Sn. During the rerun (test 1a), the charge required an additional 237 ft³ of gas for the 190-minute period to reach test temperature and 149 ft³ for the 2-hour holding period. Adding the 149 ft³ to the 490 ft³ consumed for test 1 gives 639 ft³, the estimated total gas consumption had test 1 been continued the additional 2 hours instead of interrupting for analysis, then reheating.
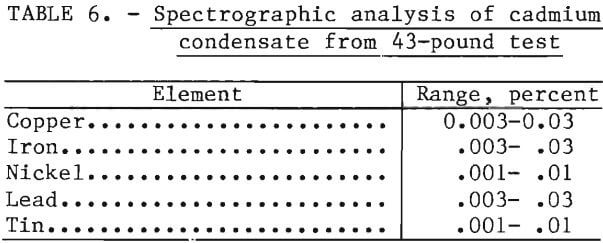
Cadmium Emissions
Cadmium emissions were caused by small leaks that would occasionally occur in the system and minor amounts from the exhaust port of the Cd condenser. Flue dust samples were taken for cadmium analysis using a modified EPA sampling system. The apparatus consisted of a sampling probe, glass filter, impingers, wet test meter, and a vacuum pump. Isokinetic samples were taken during the duration of the test. The filter assembly used glass fiber filters capable of retaining 99.98 percent of particulates down to 0.3 microns. The first two impingers contained 100 ml of 8N-HNO3, the third impinger was empty, and the final impinger contained activated alumina desiccant. Analyses of the 8N-HNO3 test solutions and a blank solution showed no cadmium in the solutions. All of the cadmium was effectively trapped on the glass fiber filters. A blank test was run with no charge in the retort and 1.58 mg/m³ was detected in the flue gas. Apparently, the cadmium emissions were caused by residual cadmium in the main fire box surrounding the retort which had been deposited from previous leaks and cracked crucibles. The cadmium emissions in table 7 have been corrected by subtracting the cadmium emission produced during the blank test. At the present time, there are no EPA source emission standards for cadmium. The test numbers correspond to the three 25-pound tests in table 4 and for the final 43-pound test. Ambient air samples in the work area were below the new recommended NIOSH standard of 40 µg Cd/m³.
Fuel Gas Consumption
The natural gas consumptions shown in tables 3, 4, and 5 were compared on the basis of charge size and total test time. These data are shown in table 8. Total test time includes the heat-up and the holding period at test temperature. During commercial processing, even in batch operations, furnaces would be charged hot to minimize fuel consumption.
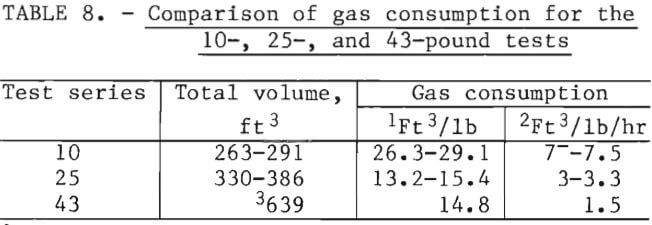
The efficiency of fuel usage was significantly improved for the 25- and 43-pound tests compared to the 10-pound tests. Additional fuel savings by further upscaling with the present retort design is not indicated from these data. In other words, the gas consumption per pound of charge at test temperature was the same for both the 25- and 43-pound tests. The burner and retort design were not investigated in this study for optimization of fuel consumption and charge throughput. These fuel consumption data are included only as a relative indication of increased fuel efficiency with increased furnace charge for the particular retorts used.
Further Research Needs
Fusion
Different degrees of fusion occurred when whole versus shredded plates were used. Apparently, the large flat surface of the whole plates gives better surface-to-surface contact causing high fusion whereas the shredded plate particles are jagged and only have “point” contact. Additional research is needed to determine the effect of KOH concentration, particle size, amount and type of carbon, degree of packing sample in retort, and addition of additives on the cause of fusion. Although the amount of fusion observed during these tests did not appear to be a major concern, more data on means to minimize fusion should aid in commercial design of such a system.
Furnace Design
Although the gas-fired furnace is the easiest to operate, it may not be the best type for this material because of poor heat transfer or conduction. This was indicated by the slow heat-up periods and excessive temperatures at the periphery of the sample. An induction furnace should give a more uniform heat because of the better coupling between the sample and the induction coil. A different type of crucible material may help with the heat transfer. Silicon carbide material gives excellent heat transfer, but in a reducing atmosphere the nickel will be reduced and react with the silicon carbide if the temperature reaches 1,200° C. Additional work could be done on the type of retort material.
Cadmium Condenser
To eliminate or reduce cadmium emission from the condenser, several modifications could be made. First, a larger condenser should be used which would allow the fine cadmium particles to settle. The larger condenser would reduce the velocity of the carbon dioxide and water vapor which act as carrier gases. Second, the condenser should be in a vertical position to allow for settling of the particles. Third, no insulation should be used. This reduces the temperature which in turn reduces the vapor pressure of the cadmium. Fourth, the sample should be dry to eliminate some of the water that acts as a carrier gas. Fifth, a proper baffle design would also reduce the cadmium emissions.
One of the major weaknesses of the system was the cement seal between the condenser and the retort. The cement that was selected (Fiberfrax) had good insulating properties, but was slightly porous. If any blockages occurred in the exhaust system, cadmium immediately leaked through the cement causing cadmium oxide fumes. Other types of cements should be tried.
Conclusions
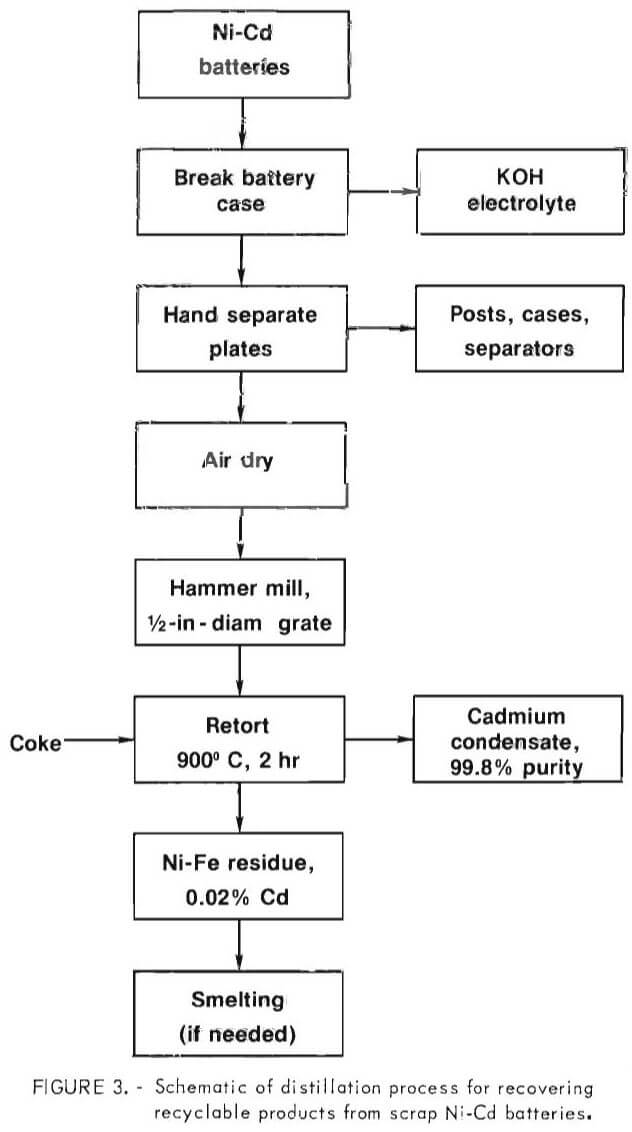
A schematic of the recycling process is shown in figure 3.
Results on retort charges ranging from 10 to 43 pounds have shown that two marketable products can be recovered from scrap Ni-Cd batteries. Based upon the data and visual observations, the following optimum conditions were defined:
- The retort charge must be heated to a minimum of 900° C for 2 hours to produce a residue with less than 0.02 percent cadmium.
- A minimum of 2.5 percent carbon is needed for effective distillation and removal of cadmium from the charge.
- The scrap battery plates must be shredded to reduce fusion in the residue.
- The reduction- distillation can be effected at atmospheric pressure and vacuum is neither required nor recommended.
Recovery tests were not conducted on the battery connecting posts. Only a small amount of grid and paste material was left on them, and it is felt that the posts could be smelted to produce a stainless steel product. The battery cases could be fragmentized, washed, and sold as a plastic.
Further work on the pyrometallurgical design would better define the parameters for optimizing the recycling process.