Recently, a number of techniques of recovering Palladium have been developed. For example, leaching of platinum group metals from spent automobile catalytic converters using halogen salt with oxygen was developed.
Ammonia is an inexpensive lixiviant used in leaching precious metals such as silver and gold. Since palladium has some similar chemical properties to silver, it is believed that ammonia leaching is applicable to palladium. As part of research activities seeking for alternative leaching of palladium, this study was initiated to examine the feasibility of ammonia leaching for recovery of palladium. A rotating disk of palladium was used in the study to investigate the dissolution behavior of palladium in ammoniacal solutions. Experiments were conducted in an autoclave at elevated temperatures with oxygen.
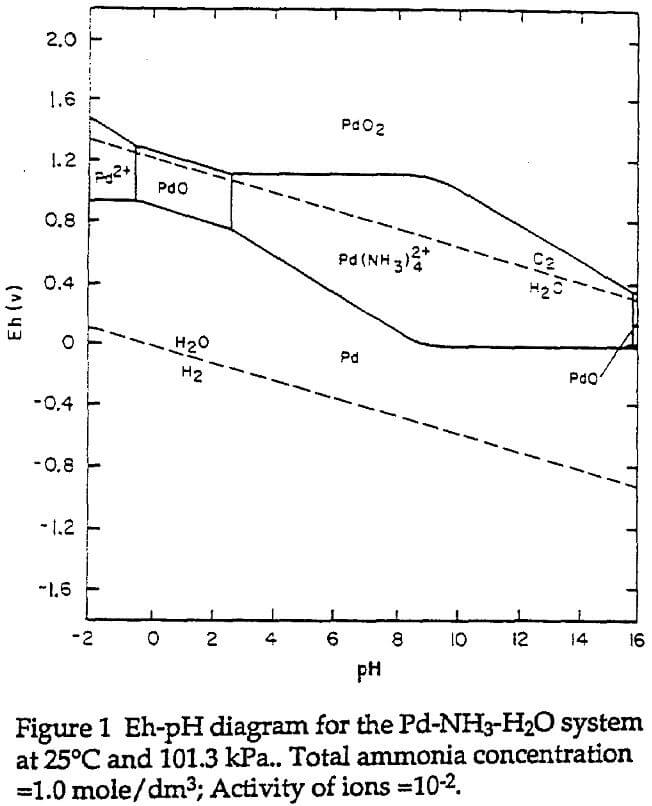
Like silver, palladium can coordinate with ammonia to form ammine complexes. These palladium ammines are soluble in aqueous solutions. The leaching chemistry can be obviously illustrated in terms of a thermodynamic stability diagram such as potential (Eh) -pH diagram. Figure 1 is an Eh-pH diagram of the Pd-NH3-H2O system at 25°C and for a total ammonia concentration of 1.0 mole/dm³ and an activity of the soluble species of 10 -2. This has been constructed by the authors based on thermodynamic data from references.
Dissolution experiments were carried out in a 1000ml autoclave made by Parr Instrument Co. USA, model No. Parr 4521M. The autoclave is equipped with a magnet stirrer of which rotating speed is set by a Parr 4841 controller. The desired temperature was controlled within ±2°C.
The rotating disk technique was used in the study. A palladium metal disk of 99.9% purity with diameter 2.4 cm and surface area 4.52 cm² was mounted to a teflon setting (diameter 3.3 cm) which was attached to a vertical shaft rotated by the magnet stirring device. Prior to leaching, the palladium disk was polished and then assembled to the stirring device.
Leaching solution of 400 ml was used in each experiment and was prepared by dissolving known amounts of reagent grade chemicals in distilled water. Before leaching experiment was conducted, the leaching solution was placed in a glass vessel and then placed in the autoclave. The autoclave was sealed and heated to a desired temperature. At the same time, the disk rotation started. After the desired temperature was reached, 99.9% pure oxygen gas was injected into the autoclave until the desired pressure was achieved.
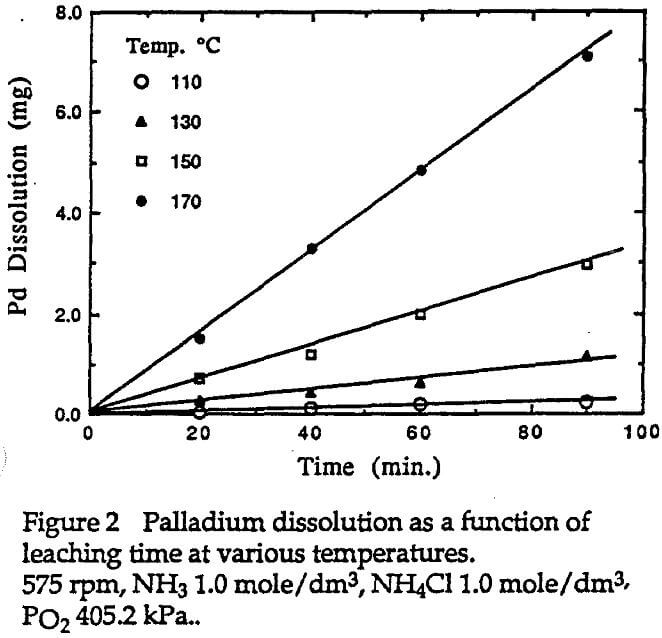
The leaching time was taken into account when oxygen was introduced. About 15 ml liquid samples were withdrawn at various time intervals for chemical analysis. The palladium concentration of the solution sample was analyzed by a Perkin-Elmen atomic absorption spectrophotometry. The volume change caused by sampling and Pd in the sample solutions were taken into account, when the calculations of the Pd dissolution were made.
It was found that the dissolution rate of palladium was so slow at ambient temperature that it could not be measured. Therefore, all dissolution experiments were carried out in an autoclave at higher temperatures so that the dissolution rate would be significant. The amount of dissolved palladium was found to be proportional to leaching time as indicated by Equation 2. The dissolution rate can be obtained based on the linear relationship of the amount dissolved with leaching time. More details of the experimental results are discussed as follows.
Experiments were conducted to examine the effect of temperature on the dissolution of palladium in ammoniacal solutions. It was found that at temperatures below 100°C, the amount of dissolved palladium into the solution was too little to be detected. At temperatures higher than 110°C, the dissolution was significant as shown in Figure 2. It is obvious that higher temperatures will result in a greater dissolution of palladium.
The dissolution behavior of palladium in ammoniacal solutions has been investigated. It was found that the dissolution was very slow at temperatures below 100°C but was significantly improved at higher temperatures, despite the thermodynamics of the dissolution reaction suggesting that palladium metal can dissolve in ammoniacal solutions at room temperature.
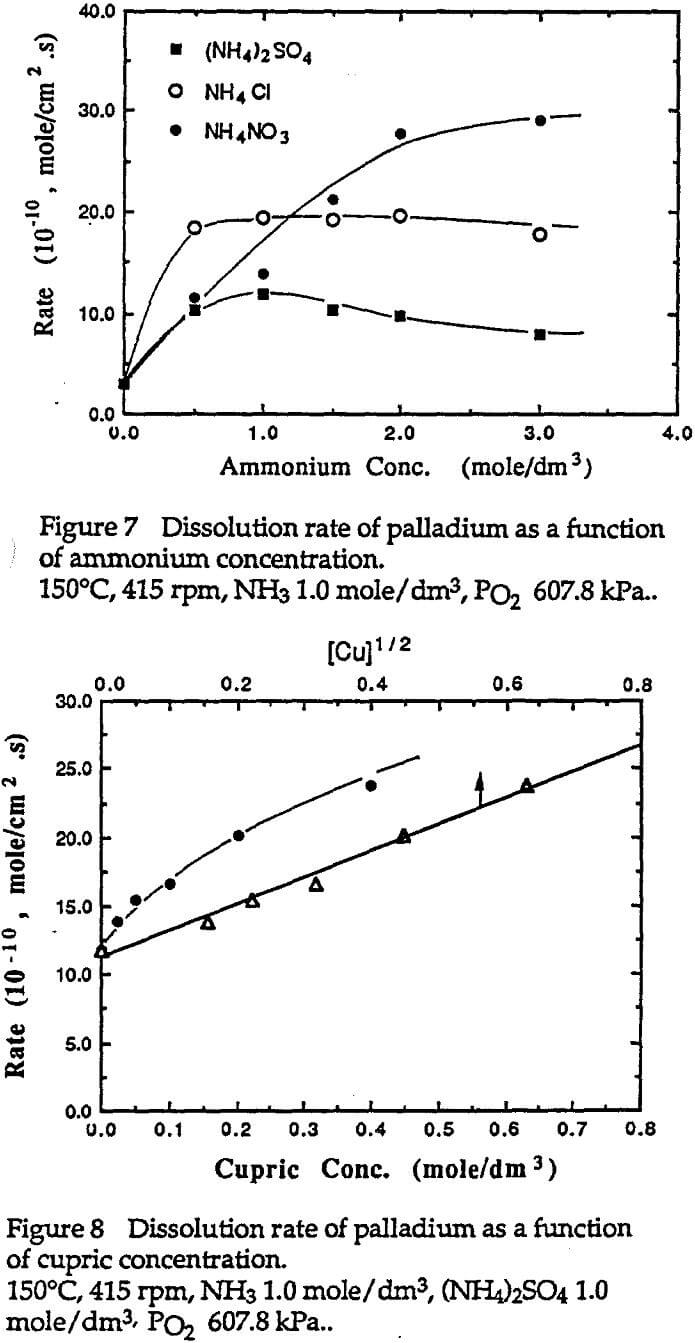
The dissolution of palladium in ammoniacal solutions was found to be chemical reaction controlled with an apparent activation energy of 69.7 kJ/mole. The dissolution rate was first order with respect to the partial pressure of oxygen and ammonia concentration. The presence of ammonium resulted in increase of the dissolution rate. However, anions and metal cations had different effects on the dissolution rate. Sulfate had a negative effect on the rate, while nitrate had a positive effect. Cupric ammines or other ions with oxidation power acted as oxidant and had a positive effect on the dissolution of palladium.

