Table of Contents
The increasing demand for titanium minerals, especially rutile, has sparked interest in the abundant domestic titanium resources of the United States. Although 93 percent of the rutile used in this country is imported, there are porphyry copper deposits in the Western United States that constitute a very large potential rutile resource.
Rutile is used to manufacture titanium pigment, an ingredient used in surface coatings. Because of its high refractive index, rutile imparts whiteness, opacity, and brightness to paints. These same qualities also make it useful for paper coatings or as paper filler. Another major use of rutile is in the manufacture of titanium sponge. In aerospace applications, titanium metal is useful because of its high strength-to-weight ratio. Other uses for rutile include welding-rod coatings, ceramic and glass formulations, carbides, and special alloys.
In 1953, a survey by the U.S. Geological Survey found that most of the western prophyry copper deposits then being mined contained TiO2 in amounts ranging from 0.30 percent to 0.75 percent. The San Manuel copper deposit in Pinal County, Ariz., has nearly 1 billion tons of porphyry copper ore reserves that contain 0.75 percent copper. This ore also contains 0.75 percent titania.
In order to realize the potential value of porphyry copper ores as a source of rutile, a feasible method for recovery must be developed. However, a search of the literature failed to disclose any information on the recovery of titania from porphyry copper tailings. In this report, a description and summary of results are presented of batch-scale studies that were designed and carried out by the Bureau to evaluate the feasibility of rutile recovery from porphyry copper tailings.
Minerals recovery research such as this is a part of the Bureau’s overall effort to maintain an adequate supply of minerals to meet national economic and strategic needs. Such research serves to improve mineral resources technology, assure more effective use of domestic mineral resources, and maintain a continuing national supply of mineral commodities.
Description of The Tailings Sample
The Bureau obtained a sample of porphyry copper tailings from a mill that treats ore known to contain rutile. The sample was collected from the mill tailings discharge. Prior to shipment, the sample was thickened until it contained about 65 percent solids From the original sample, representative samples were derived for testing at the laboratory by the following procedure:
- As-received material was diluted until it contained about 50 percent solids;
- the resultant slurry was agitated until it was considered homogeneous;
- the slurry was screened to remove any tramp 35-mesh oversize material (mainly woodchips); and
- individual test samples were siphoned from the slurry while the slurry was in agitation.
The tailings sample was then analyzed to determine its chemical composition and particle-size distribution. The results of these analyses are shown in table 1. The distribution data show that nearly half of the TiO2 content was found in the minus 400-mesh fraction of the sample.
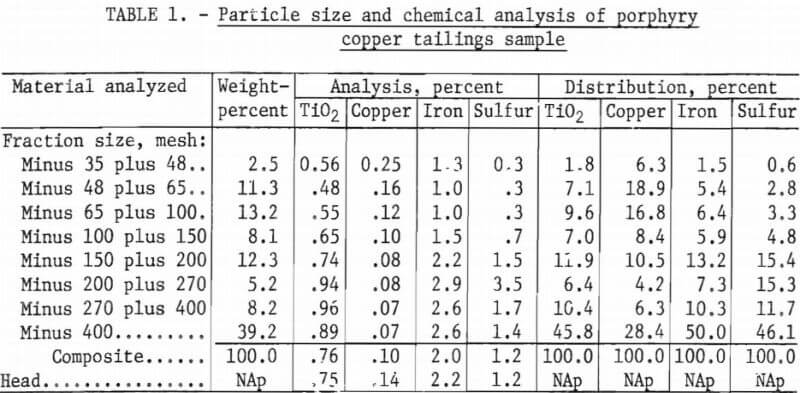
Microscopic examination showed that in the plus 200-mesh fractions, about 80 percent of the rutile was locked with other minerals. But in the minus 200-mesh fractions, the proportion of locked rutile to that which was liberated was nearly reversed; more than 70 percent of the rutile was liberated.
The principal mineral components of the sized fractions were feldspar, quartz, pyrite, calcite, and biotite. There were lesser amounts of titanium-bearing minerals—mainly rutile, some muscovite, and chlorite, plus minor amounts of zircon, chalcopyrite, and molybdenite. To further characterize the sample, a heavy-liquid analysis was done.
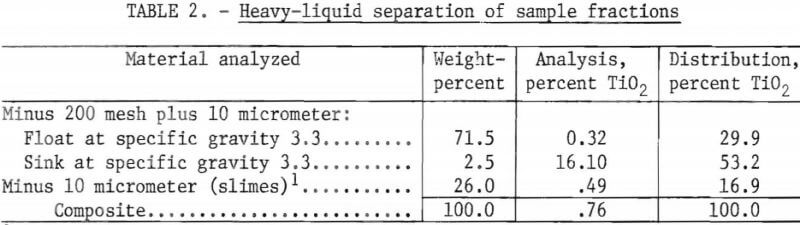
A representative sample was ground to pass a 200-mesh sieve and classified into a minus 200-mesh plus 10-micrometer fraction and a minus 10-micrometer slime fraction. The minus 200-mesh plus 10-micrometer fraction was treated using the heavy liquid diiodomethane at a specific gravity of 3.3 to obtain a concentration of the titanium-bearing minerals. Table 2 shows the results of the heavy-liquid separation. This study demonstrated that approximately 53 percent of the total TiO2 is potentially recoverable from tailings ground to minus 200 mesh. The study also showed that approximately 30 percent of the total TiO2 cannot be considered liberated at minus 200 mesh. The remaining TiO2—approximately 17 percent of the total—was contained in the minus 10-micrometer fraction.
Grinding the sample to pass 325 mesh did not result in any increased liberation of TiO2. Mineralogical studies of a sample ground to minus 325 mesh revealed that much of the rutile it contained was present in a fine crystalline form. Furthermore, the locked rutile is so closely associated with other minerals that grinding to an even finer size would not only be uneconomical, but would not be expected to result in significantly greater liberation of the rutile.
Preliminary Beneficiation Studies
Beneficiation techniques considered for concentrating the titanium-bearing minerals in the tailings sample were gravity separation, magnetic separation, and flotation. The characterization studies showed that the material had to be ground to minus 200 mesh to achieve a significant degree of liberation. This fine particle size limited the effectiveness of conventional gravity or magnetic separation methods.
Although the liberation size of rutile hampered gravity concentration, several gravity techniques were investigated to determine if a low-grade concentrate could be produced that would yield a high-TiO2 recovery. Techniques studied used spirals and several types of tables. In addition to testing these techniques using the as-received sample, some samples were ground before testing to minus 200 mesh to liberate more of the rutile. TiO2 recovery from the spiral concentrates, which contained from 0.96 percent to 1.2 percent TiO2, was only 35 percent. Tailings left from treating the spiral concentrates contained 0.5 percent TiO2. Table-concentrating tests were conducted using a regular table deck, a slime deck, and a Bartles-Mozley slime-concentrating table. All three techniques produced table concentrates that contained from 1.8 percent to 3.9 percent TiO2. Using these three techniques, only 23 percent to 42 percent of the TiO2 was recovered. The TiO2 content of the table tailings could not be made lower than 0.5 percent.
Magnetic separation tests were conducted on as-received samples and on material ground to minus 200 mesh. The tests were carried out in wet, high-intensity magnetic separators. The highest grade concentrate obtained was 2.5 percent TiO2. Recoveries did not exceed 40 percent TiO2.
Since gravity concentration and magnetic separation did not produce a low-grade concentrate at a high-TiO2 recovery level, the major thrust of this investigation was directed toward flotation.
Flotation
A basic method of flotation was devised, drawing upon the results of the beneficiation studies. Using this method, preliminary flotation tests were conducted on tailings samples that were ground to minus 200 mesh and deslimed at 10 micrometers. Subsequently, flotation tests were conducted on as-received samples that were also deslimed at 10 micrometers—but not ground. The purpose of the flotation tests was to determine whether rutile recovery is more feasible from ground tailings samples or from as-received samples.
Ground Tailings
250-Gram-Batch Tests
A 250-gram-batch flotation cell was used for the preliminary flotation tests. To prepare a sample for flotation, the material was screened on a 200-mesh sieve. The oversize, which amounted to 47.4 weight-percent of the sample, was stage ground in tap water to minus 200 mesh. The ground material was then added to the material that was previously sieved to minus 200 mesh, and the pulp was deslimed by decantation at 10 micrometers (equivalent quartz diameter).
A residual rougher sulfide concentrate consisting mainly of pyrite, was floated at pH 8.5, using sodium isopropyl xanthate as a collector. A water-soluble alcohol was used as a frother. The sulfide rougher tailings were filtered, repulped, and conditioned using 0.5 pound of hydrofluoric acid per ton of ore as a selectivity-assisting agent. Twenty pounds of sulfuric acid (H2SO4) per ton of sample ore were used to adjust the pH to 2.5. Tests showed that the best results for rutile flotation were obtained in the pH range from 2.0 to 3.0. A rougher rutile concentrate was floated using 1.0 pound petroleum sulfonate per ton of ore as the collector. The rougher rutile concentrate was cleaned four times. Each cleaning stage required about 0.3 pound of H2SO4 per ton to maintain the pulp pH at 2.5. Table 3 shows the results of a representative test of this preliminary flotation investigation.
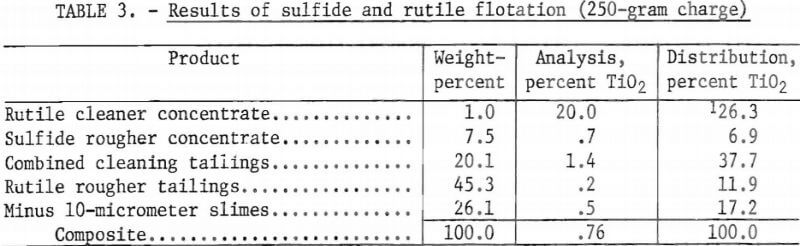
As the data in table 3 indicate, the final rutile cleaner concentrate analyzed 20.0 percent TiO2, or 49.3 percent of the recoverable TiO2, with a recovery of 26.3 percent of the total TiO2. “Recoverable TiO2” refers to the percentage of TiO2 that is potentially recoverable, as determined by the heavy-liquid analysis described in the “Description of Tailings Sample” section.
Reaction of the H2SO4 with the carbonate minerals present in the sample resulted in excessive acid consumption and considerable variation of the pulp pH during flotation. Attempts to float rutile with a fatty acid in an alkaline circuit were unsuccessful. Therefore, the carbonates were floated in an alkaline circuit prior to rutile flotation. The carbonates were floated using 0.5 pound of sodium oleate per ton of ore as a collector and 0.4 pound of dextrin per ton of ore for depression of rutile. After removing the carbonates, the H2SO4 required to adjust the pulp pH to 2.5 was reduced to about 5.0 pounds per ton of ore, and the pH of the pulp during rutile flotation became stable. Pulp during these flotation tests was maintained at ambient temperatures of 24° to 25° C. The rougher rutile concentrate was cleaned three times.
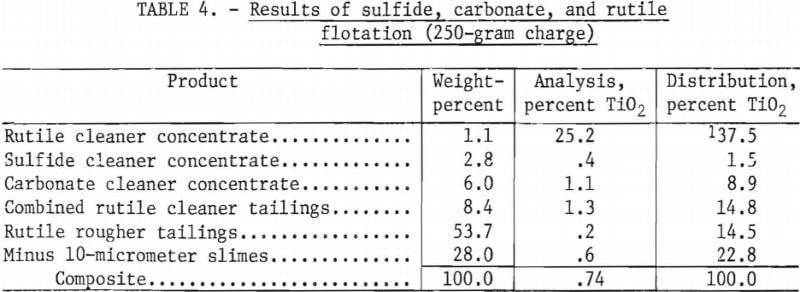
Table 4 shows the results of a representative test in which the carbonates were removed prior to rutile flotation. A reagent schedule for this test is shown in table 5. In this representative test, the sulfide and carbonate rougher concentrates were each cleaned once. The purpose of this cleaning was to minimize the amount of TiO2 retained by the concentrates. Failure to clean the concentrates would have allowed all the TiO2 retained in them to be lost, but through cleaning, some of this TiO2 was recovered. Preliminary flotation tests showed that the sulfide concentrate—before cleaning—retained 6.9 percent of the total TiO2 (see table 3). After cleaning, however, the amount of TiO2 retained in the sulfide concentrate was reduced to 1.5 percent of the total. The cleaner tailings from the sulfide and carbonate concentrates were added to the subsequent flotation feed.
The data in table 4 showed that flotation of rutile from ground tailings, after the sulfides and carbonates were removed, yielded a rutile concentrate grading 25.2 percent TiO2. The process recovered 37.5 percent of the total TiO2 and 70.4 percent of the recoverable TiO2.
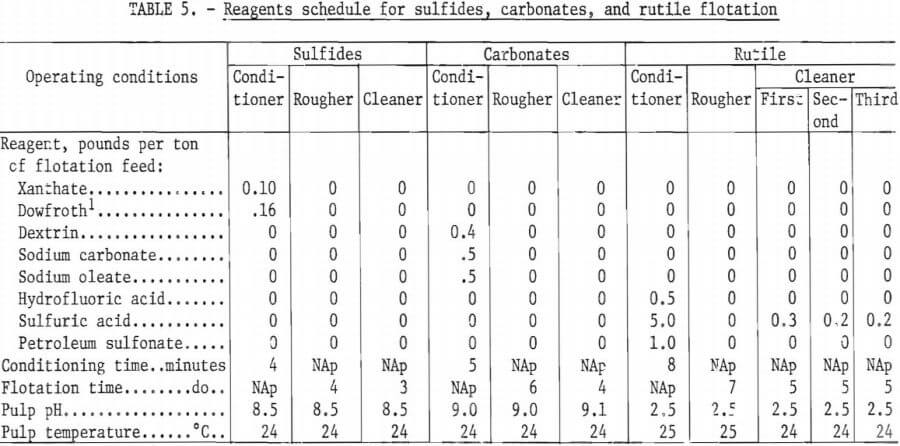
2,000-Gram Batch Tests
The previous series of tests used a 250-gram flotation cell for all rougher and cleaning operations. However, in that series of tests, pulp density in the rutile circuit was too low during the cleaning stages for efficient flotation. Therefore, an additional series of tests was conducted using a 2,000-gram flotation cell for all rougher flotation operations. For the cleaning operations, a 250-gram flotation cell was again used. When the two flotation cells were used in this way, the amount of solids in the second, or last, rutile cleaning step was about eight times greater than in the previous series of tests. All other test variables, such as particle size, conditioning and flotation time, pulp temperature and pH, and amount of reagents, remained the same as in the previous test series. Table 6 summarizes the results of a representative test of this series.
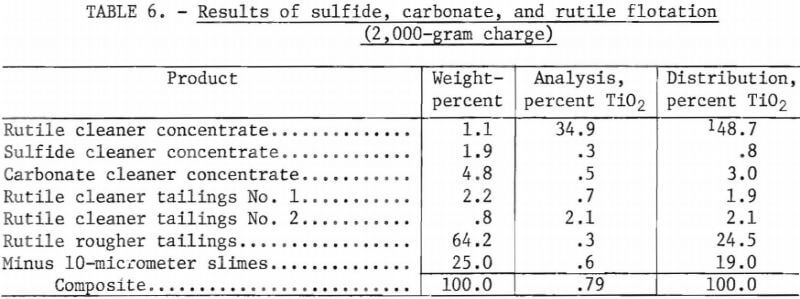
As indicated by the data in table 6, the higher pulp density in the cleaning stages achieved—as expected—a higher grade and higher level of rutile recovery. An important finding was that the rougher concentrate had to be cleaned only twice to obtain a concentrate grade of 34.9 percent TiO2. When the larger flotation cell was used for rougher operations, recovery of TiO2 increased to 48.7 percent of the total and 91.4 percent of the recoverable TiO2. Each of these figures is considerably larger than the corresponding figures obtained for the 250-gram-batch tests.
Copper Recovery
Besides the 0.75 percent TiO2 contained in the tailings sample, there was also 0.14 percent copper present. The sulfide concentrates that were floated prior to carbonate removal and rutile concentration analyzed about 1.5 percent copper, representing a copper recovery of about 20 percent. Attempts to upgrade the copper contained in the sulfide concentrates were not successful, and the results did not appear to justify separate sulfide and carbonate flotation steps for copper recovery.
Flotation of a Bulk Concentrate
Studies were conducted to determine if a bulk sulfide-carbonate concentrate could be floated and to determine what, if any, effect this procedure would have on rutile concentration. The studies showed that it was feasible to float a bulk sulfide-carbonate concentrate. Furthermore, this procedure did not have any adverse effect on the rutile-flotation stage. In a continuous operation, bulk flotation of these two products would require less equipment than would be required for selective flotation.
Studies of Optimum Collector Quantity
A series of tests was conducted to establish the optimum amount of collector to be used for the best rutile grade-recovery combination after removal of the bulk sulfide-carbonate concentrate. The amount of petroleum sulfonate, the rutile collector, was varied from 0.5 to 2.0 pounds per ton of tailings. All other flotation variables remained constant for this test series. Results of these tests are shown in table 7, and a summary of test conditions and the reagents schedule for these tests are shown in table 8.
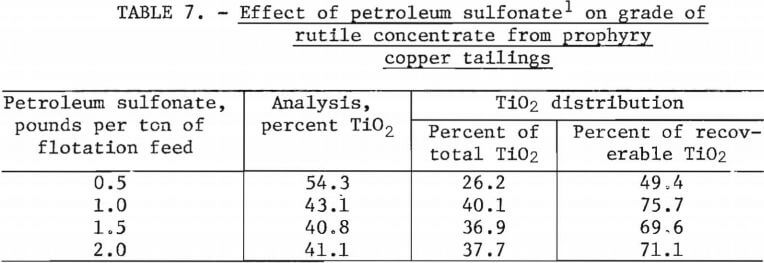
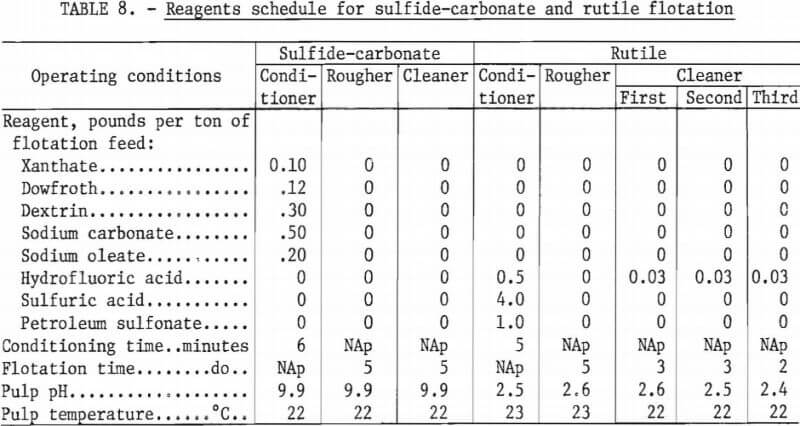
The data in table 7 indicated that the use of 1.0 pound of petroleum sulfonate per ton of ore resulted in the best grade-recovery combination for rutile concentration. When the amount of collector was decreased to 0.5 pound per ton of ore, a concentrate grading 54.3 percent TiO2 was obtained, but TiO2 recovery was low. Increases in collector consumption to 15 and 2.0 pounds per ton of ore resulted in decreases in both grade and recovery of TiO2. As shown in table 8, pulp in this series of flotation tests was maintained at ambient temperatures of 21° to 23° C.
As-Received Tailings
The basic flotation scheme utilized for the previous tests required grinding the copper tailings to minus 200 mesh. Subsequently, flotation studies were conducted using tailings that were not ground (as-received tailings).
Procedure
The as-received sample was classified into two fractions, a minus 35-mesh plus 10-micrometer sands fraction and a minus 10-micrometer slimes fraction. The tramp 35-mesh oversize material had been previously screened when the individual test samples were prepared. Using the minus 35-mesh plus 10- micrometer fraction as flotation feed, a bulk sulfide-carbonate concentrate was floated and cleaned once without additional reagents. The bulk rougher and cleaner tailings were combined, thickened to about 35 percent solids, conditioned with the necessary reagents; and a rutile rougher concentrate was floated. The rutile rougher concentrate was cleaned three times. The same reagents schedule as shown in table 8 was used.
Results
Results of a representative test using as-received tailings are shown in table 9. Table 10 compares a rutile concentrate obtained from tailings ground to minus 200 mesh with a rutile concentrate obtained from the as-received material. For each of the two concentrates, this table shows weight-percent, total TiO2 recovered, the TiO2 grade, and the amount of TiO2 that is potentially recoverable from each sample.
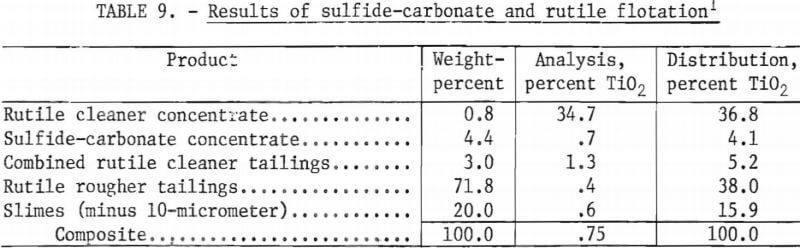
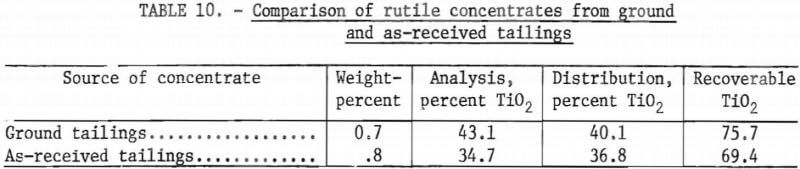
Using the as-received tailings deslimed at 10 micrometers as flotation feed, it was demonstrated that a rutile concentrate of 34.7 percent TiO2 could be obtained with recoveries of 69.4 percent of the recoverable TiO2 and 36.8 percent of the total TiO2. Rutile flotation without grinding the plus 200-mesh material appears to be the most promising beneficiation technique. Although the tailings that were ground resulted in greater TiO2 recoveries than did the as-received tailings, the differences in recoveries were too small to justify the additional expense of grinding.
Millsite Testing
Because Arizona’s water resources are limited, the quality and quantity of available water are deficient for some industrial uses. Consequently, reclaimed water is used for some industrial applications in Arizona. The industry most seriously affected by the State’s water shortage is the mineral industry. In order to determine what effect, if any, the use of reclaimed water would have on the Bureau-developed rutile flotation technique, onsite batch-flotation tests were conducted using a tailings sample from an Arizona millsite.
At the mill where the tailings sample was obtained, 80 percent of the water used is reclaimed and recycled to the mill operation. The mill uses process water that is 80 percent reclaimed and 20 percent potable water.
Samples for the Bureau’s onsite tests were collected from the mill tailings discharge section. The flotation procedure was basically the same as was described for flotation of the as-received tailings. All test conditions remained the same, except for the water source used for flotation.
Table 11 shows the results of rutile-concentration tests that were performed using water from various sources available at the mill. Results of a test using Tuscaloosa, Ala., tap water, are also listed for comparison.
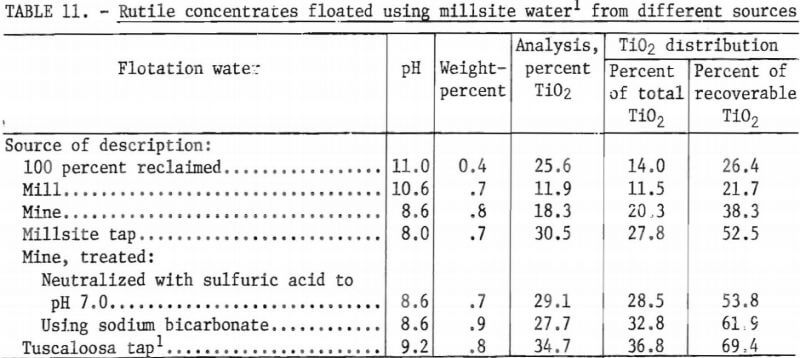
The data in table 11 indicated that the mine water was as effective for flotation as was millsite tap water (the mine water was neutralized with H2SO4 to pH 7.0 and/or treated with sodium bicarbonate before flotation). Using these two sources of water resulted in the highest TiO2 grade-recovery combinations achieved at the millsite. However, the flotation results using mine water and millsite tap water were lower than those obtained in batch tests at Tuscaloosa using local tap water.
The reclaimed water was highly alkaline because it contained lime. Most western porphyry copper sulfide mills operate at a pH of 11 or higher and use lime to control pH. A high-lime circuit is specifically designed to depress iron sulfides—mainly pyrite—while floating sulfide copper minerals. Thus, in the test using mill water, the addition of more xanthate collector without reconditioning of the pyrite surface will not result in effective flotation of the pyrite. Furthermore, the high-lime circuit has a high concentration of calcium ions that will react with the calcite collector, sodium oleate, to form an insoluble calcium oleate. For these reasons, the high-lime circuit appears to present problems for flotation of both pyrite and carbonate minerals.
Electron Microbeam Analyses of Concentrates
In order to determine what mineral associations were present in the flotation products, electron microbeam analytical techniques were utilized.
Products that were analyzed were taken from a single test in which the tailings were ground prior to flotation. These products included a rutile concentrate containing approximately 45 percent TiO2, a sulfide concentrate containing 0.8 percent TiO2 and 1.5 percent copper, and a carbonate concentrate containing 0.7 percent TiO2. Results of the analyses are given below.
Rutile Concentrate
Quantitative phase analysis was not attempted because of the small and irregular grain size and complex nature of the sample. A qualitative analysis revealed that the sample contained the following minerals in the ranges indicated:
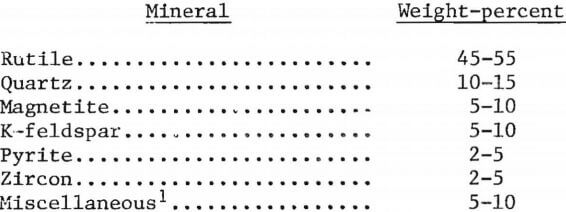
Several sets of scanning-beam photographs were obtained that depict characteristic features of typical grains found in the rutile concentrate. These characteristics can be seen in figure 1. In figure 1A, an electron backscatter image of the rutile concentrate, letters mark the location of quartz, rutile, zircon, pyrite, and magnetite grains, as explained in the caption. Iron oxide stringers can be seen crossing through rutile grain . The other four rutile grains are pure; each of these is marked with an R. There are two types of iron oxide present: one is a titaniferous magnetite (marked in fig. 1 as M1) containing about 9 percent titanium; the other (M2) is an iron oxide containing 0.5 percent titanium, 2.0 percent silicon, and 2.5 percent copper. The sample contained only a small percentage of pure magnetite. Other sets of photographs, not included in this report, showed grains of rutile mixed with K-feldspar, quartz, pyrite, and xenotime.
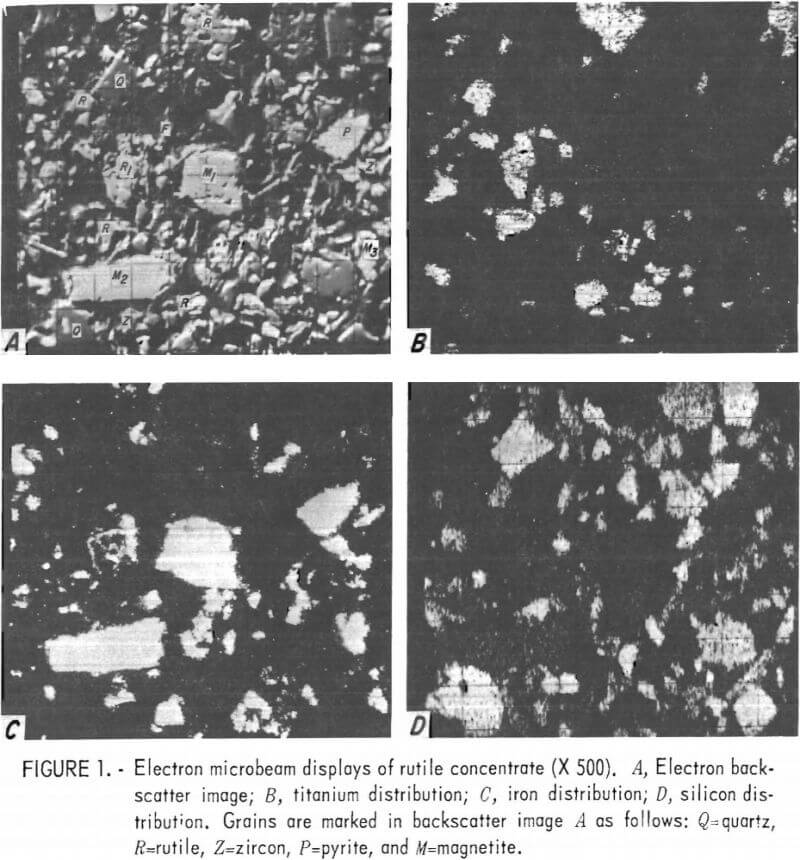
Sulfide Concentrate
Minerals identified in the sulfide concentrate and their approximate quantities were as follows:
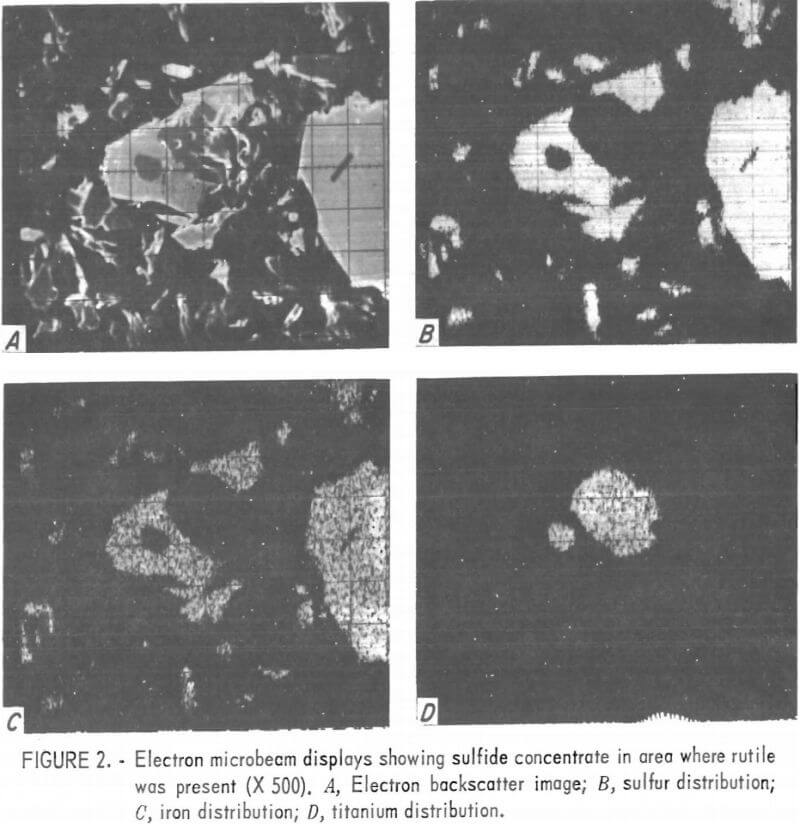
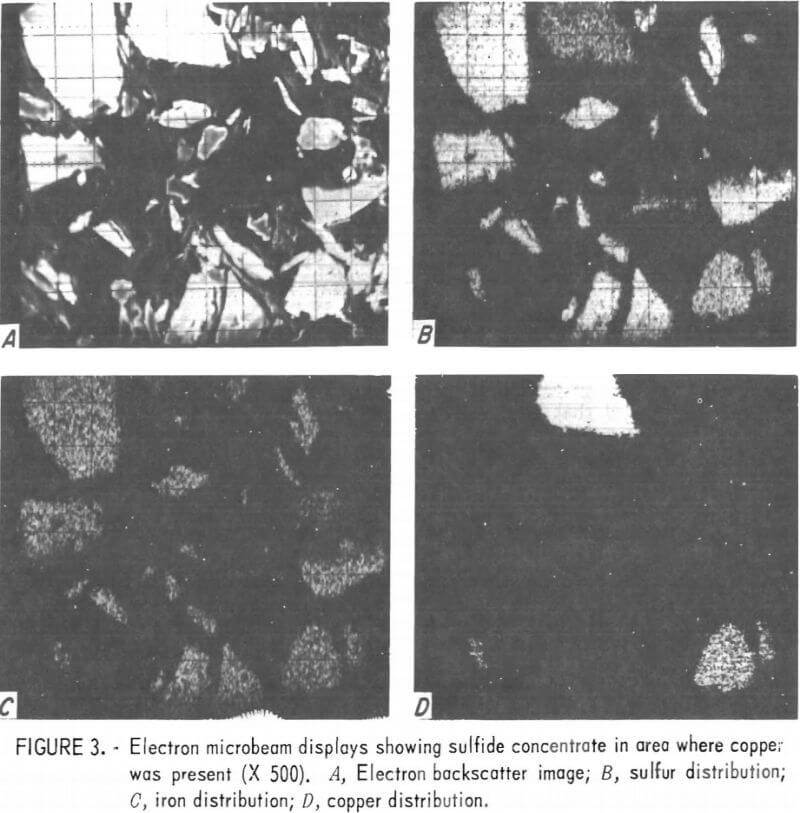
Figure 2 shows an area of the sulfide concentrate where rutile was present. Most of the rutile in this concentrate was attached to or contained within the pyrite grains. Near the center of the photograph, a typical mixed mineral grain of pyrite and rutile can be seen. Most of the remaining grains are pyrite. Figure 3 shows microbeam images of an area where copper was found; visible within this area are one bornite grain, three chalcopyrite grains, and a number of pyrite grains.
Carbonate Concentrate
The mineral composition of the carbonate concentrate, as determined by microbeam and X-ray diffraction, was as follows:

In the carbonate concentrate, titanium was found only in the rutile particles. These rutile particles were much smaller than the average particle size of the total concentrate.
Conclusions
Characterization studies of the porphyry copper mill tailings used for this research showed that the tailings material contained 0.75 TiO2. Rutile was the most abundant TiO2 mineral. Mineralogical examination of the ore after it was ground to pass 200 mesh showed that about 30 percent of the TiO2 remained locked and that nearly 17 percent of the TiO2 is lost in the minus 10-micrometer slimes. Therefore, about 53 percent of the total TiO2 can be considered recoverable. This limit appears to be low, but because of the size of the milling operation, recovery of even 40 percent of the TiO at a marketable grade would provide a significant portion of the rutile consumed annually in the United States.
Beneficiation studies showed that both gravity and high-intensity magnetic separation failed to produce either an appreciably upgraded product or a waste product.
Rutile flotation without grinding the plus 200-mesh material is feasible and appears to be the most promising technique for rutile beneficiation. A rutile concentrate grading 34.7 percent TiO2 with recoveries of 69.4 percent of the recoverable TiO2 and 36.8 percent of the total TiO2 was obtained using as-received (not ground) tailings.
Electron microbeam studies of a rutile concentrate and other flotation products showed the complex mineralogical nature of the sample, and therefore indicated the need for a complex recovery process.
Recommendations
It is recommended that further studies be made to determine to what extent the grade of TiO2 concentrates can be increased without drastically lowering recoveries. A preliminary cost evaluation should be made to determine the approximate cost of producing a ton of contained TiO2. If warranted, continuous miniplant-scale studies could be undertaken.
More testing using mine and mill water available at the millsite is necessary to determine whether either or both water sources might be suitable for rutile flotation. Treatment of these waters to remove or complex the calcium ions and an additional step to scrub the mineral surfaces are recommended.
A survey should be undertaken to determine what other tailings are available that might be potential sources of TiO2.
The Bureau of Mines conducted batch-scale tests on a sample of porphyry copper mill tailings as part of a study to determine the feasibility of rutile recovery from this source. The tailings that were tested contained 0.75 percent titanium dioxide (TiO2), with about two-thirds of the TiO2 values occurring as rutile. Mineralogical studies indicated that about one-half of the TiO2 content could be considered recoverable.
Beneficiation studies of the tailings showed that best flotation results were obtained when sulfides and carbonates were removed by bulk flotation prior to rutile flotation. Rutile flotation was most effective when a petroleum sulfonate collector was used in an acid circuit. From this basic method of flotation, two alternative procedures were developed. The first procedure was to size and grind the tailings to minus 200 mesh; deslime the total pulp; and then float the sulfides, carbonates, and rutile. The second procedure was the same, except that it did not include sizing and grinding. Flotation of the sized and ground tailings yielded rutile concentrates containing 43.1 percent TiO2 and 75.7 percent of the recoverable TiO2. Rutile concentrates floated from the tailings that were deslimed but not ground contained only 34.7 percent TiO2 and 69.4 percent of the recoverable TiO2.
