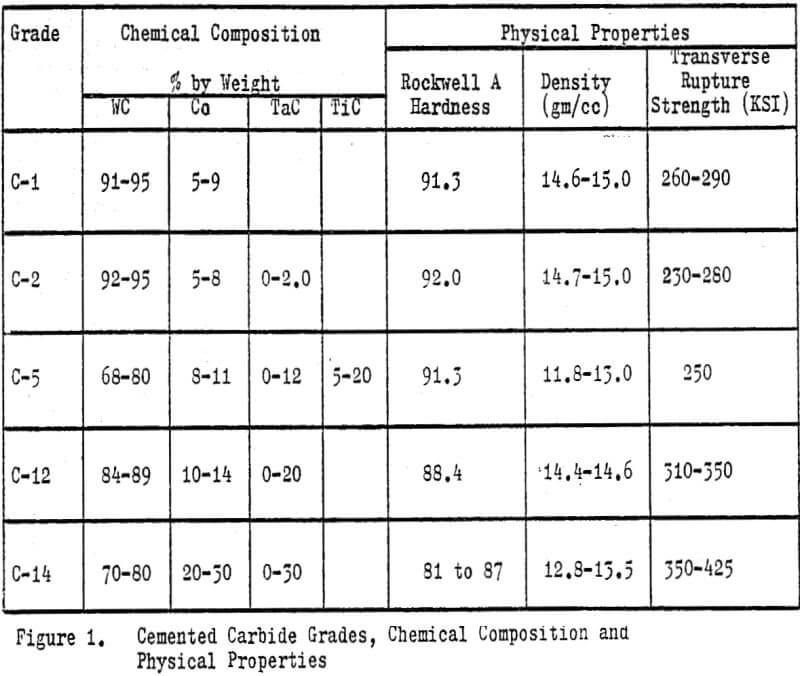
Tungsten carbide (WC) is chemically a binary compound of tungsten and carbon in the stoichiometric ratio of 93.87% tungsten and 6.13% carbon. However, industrially the term usually implies cemented tungsten carbides (CWC); a sintered powdered metallurgical product consisting of very fine grains of pure tungsten carbide bound or cemented together in a cobalt matrix. The size of the tungsten carbide grains range from ½ to 10 microns. The cobalt content can vary from 3 to 30%, but will typically range from 5 to 14%. The grain size and cobalt content determine the application or end use of a finished product. Common carbide products include cutting and forming tools, drills, abrasives, rock bits, dies, rolls, ordnance and wear surfacing materials.
Manufacturing of Tungsten Carbides
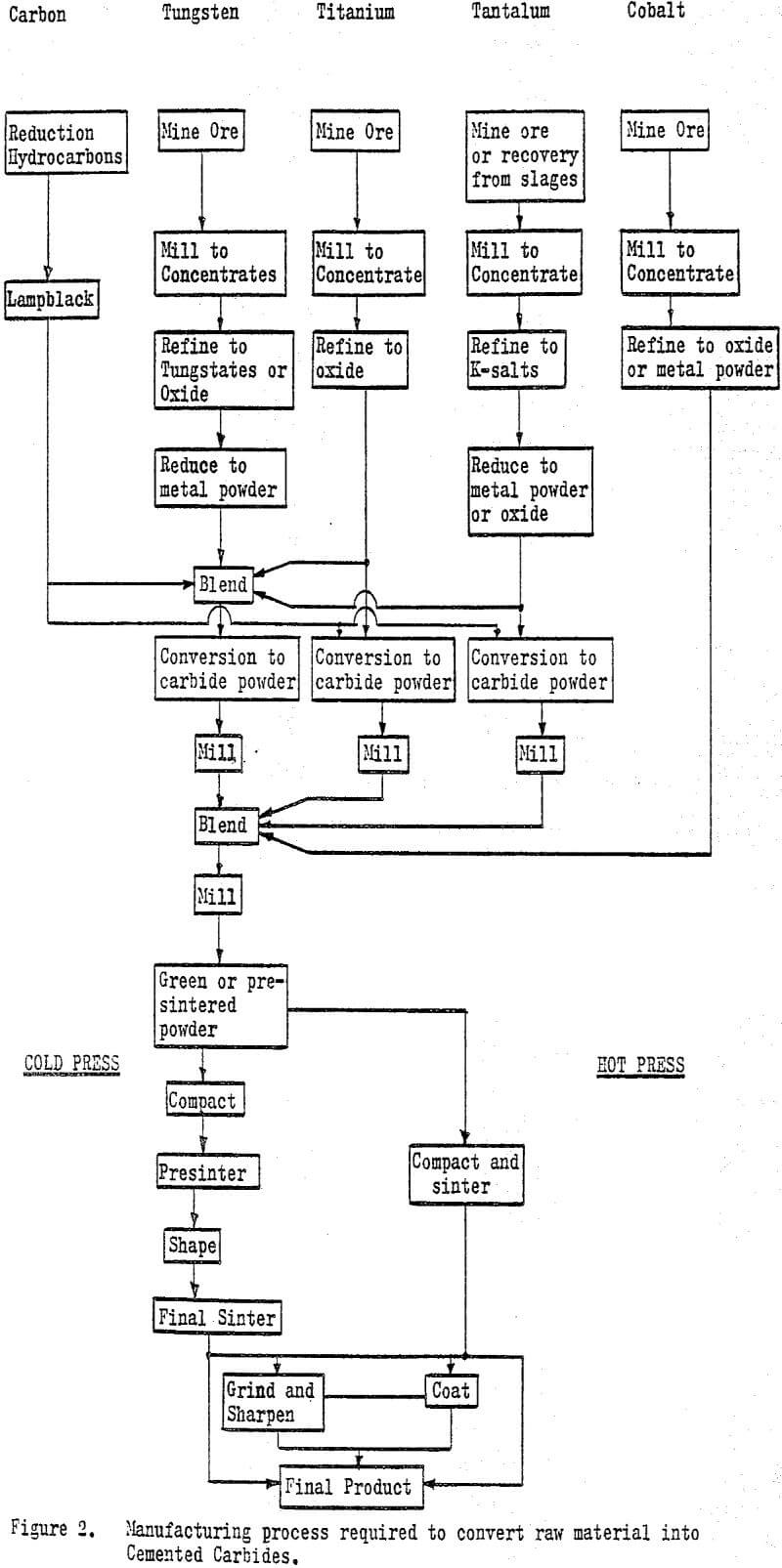
The manufacturing process for cemented carbides from mined ore to finished product is quite lengthy and complex. Tungsten and cobalt are mined and milled to concentrates. The concentrates are refined commonly to the metal oxide which is then reduced to a pure metal powder. Tungsten powder is blended with carbon (lampblack) and converted to tungsten carbide usually in induction furnaces at temperatures from 1300 to 1500° C (2400 to 2800° F).
The sintering operations can be conducted in either resistance or induction type furnaces of stainless steel construction and water cooled. Sintering is carried out under an inert atmosphere or vacuum to reduce oxidation. Hot pressing is done with resistance heated, isostatic presses.
Because of its industrial importance, moderate unit value and strategic significance, cemented tungsten carbides hare become an important recycled material. However, as with any recycling scheme, the cost to produce a material by recycling waste or scrap must be equal to or less than the cost to produce the same material from its raw components to be economically viable – all other factors being equal.
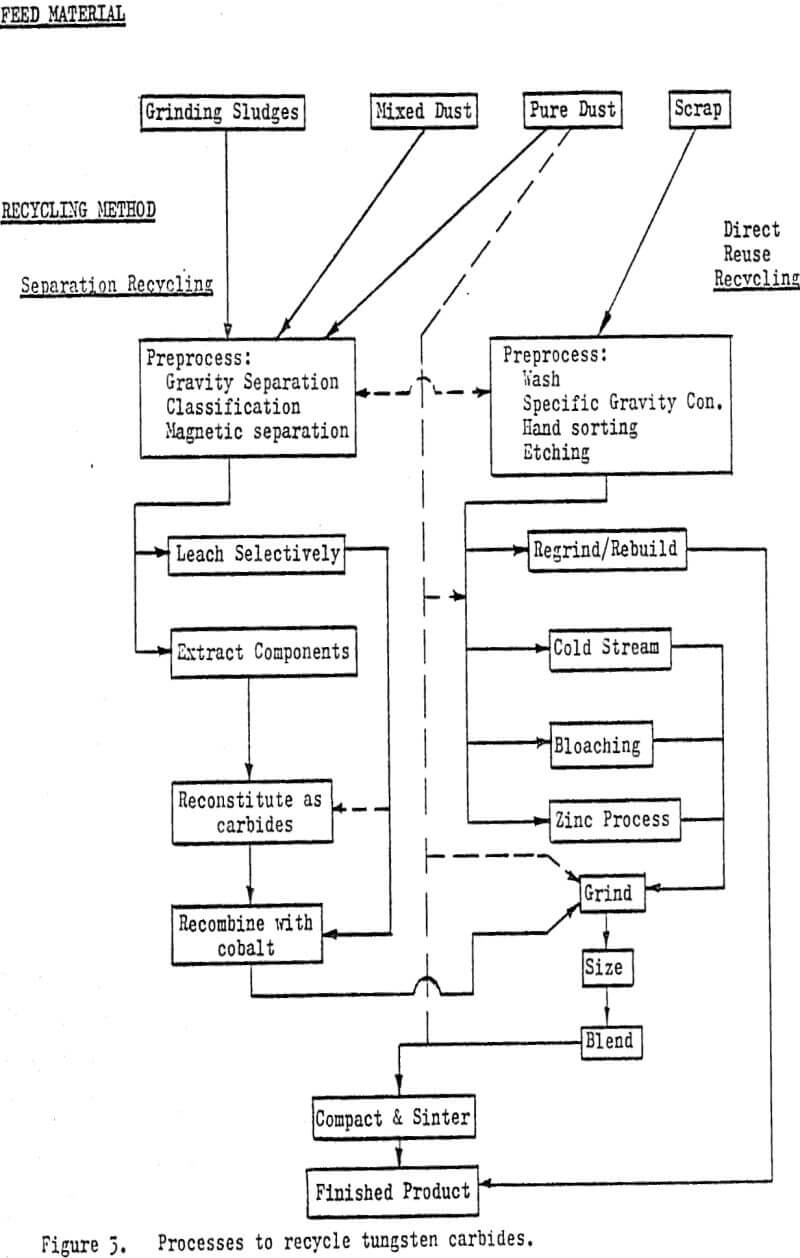
Common feed materials include sludges, pure and mixed dusts and whole or broken scrap carbides. Sludges are generated as a by-product of machining cemented carbides before the final sintering operation or grinding them after the hardening sinter. The material is usually highly contaminated, can be oxidized and may be coated or mixed with organic material.
Prior to any treatment, dusts and sludges may require upgrading or concentration. Gravity separation and classification techniques are used to remove dirt and other diluents. Magnetic separation may be used but both iron and many carbide materials are magnetic. Organic coatings can be removed in log washer or submerged trommel operations.
Those materials which are extremely oxidized or contain other chemically inert components can be recycled back to their raw metal states. There are a number of approaches used but all employ chemical recovery methods. Material may initially be chlorinated or oxidized and then alkali or acid leached. Tungsten is recovered as AFT (ammonium paratungstate) or as a tungstate (tungstic acid).
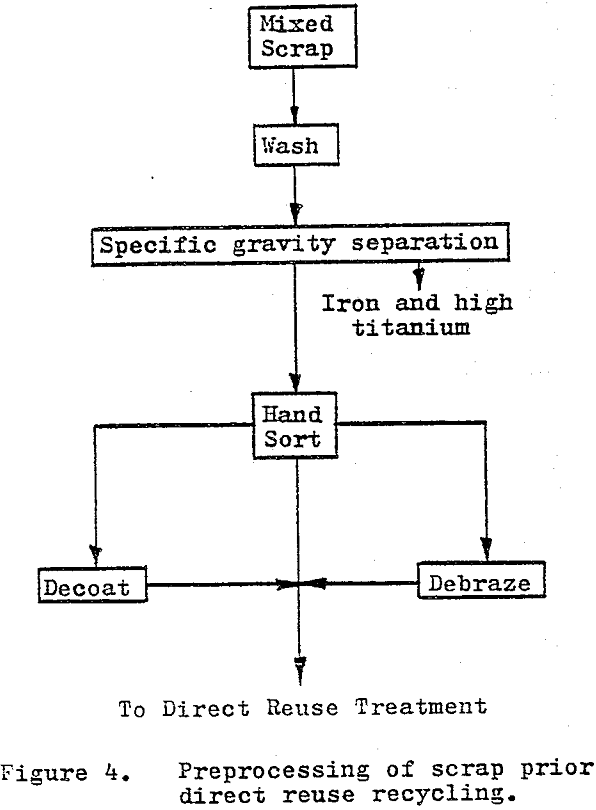
Direct-reuse recycling methods are those which do not chemically alter the material to be recycled in any permanent manner. The recycled material is returned to the carbide manufacturing sequence at a relatively advanced level and at a higher unit value than most separation recycled products.
By far the simplest and most economical approach of the direct-reuse recycling is to regrind or rebuild the individual pieces of scrap to produce a usable component (tool). Rebuilding is generally limited to resurfacing or rebuilding wear parts with “tungsten electrodes”.
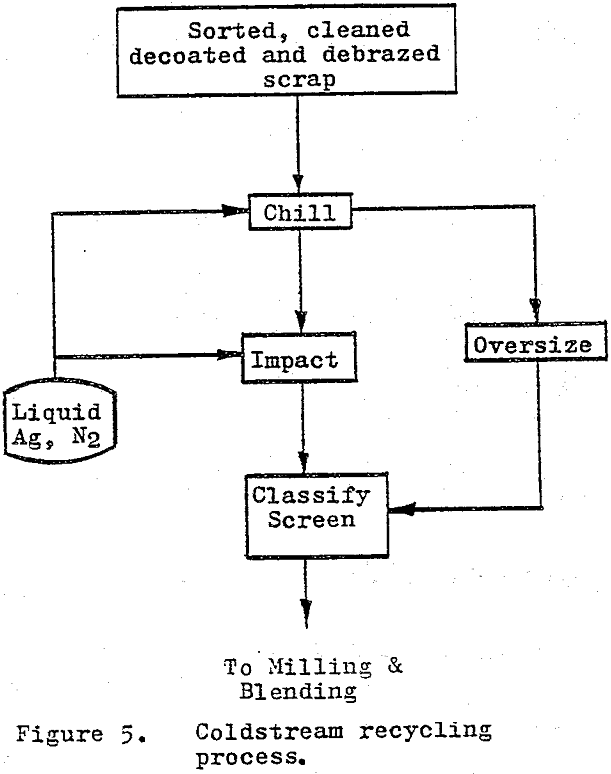
The cold stream process utilizes temperature-depression embrittlement and differential contraction rates combined with impact breaking to reduce the cemented carbide scrap to a coarse powder. 100$ minus 250 to 150 µm (60 to 100 mesh). As originally developed, the cold stream process used blasts of air to impact the scrap particles against a surface with sufficient energy as to cause fracture.
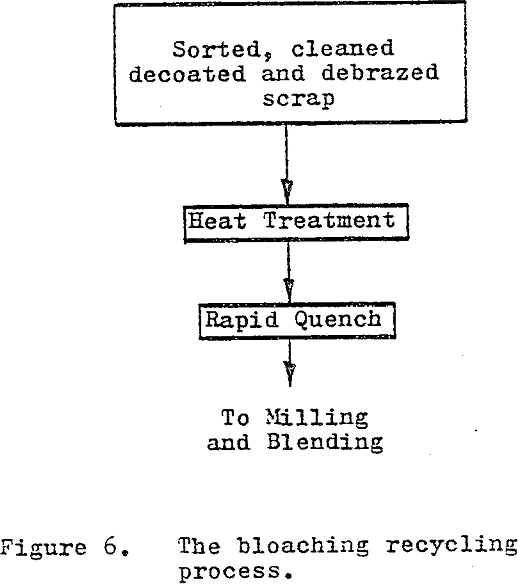
The bloaching process uses a high temperature heat treatment (1800° C – 3300° F) followed by a rapid quench to produce a material capable of being reduced to a presintered powder through conventional comminution methods. The effects of grain growth, induced thermal stress and phase transformation combined with the rapid quench, create a laminar structure with extremely brittle layers.
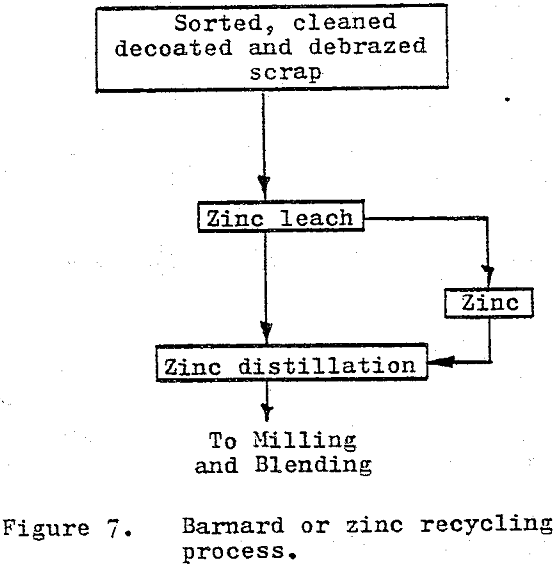
The zinc or Bernard process as it is referred to in the U.S. is based upon the solubility and associated phase changes of the cobalt in cemented carbide materials when leached with liquid zinc.
Furnaces are generally of mild steel construction with the hot zone typically insulated from the furnace shell by graphite wool. Electrodes and heating elements are graphite. The crucible areas in the cool zone are surrounded by water cooled, plate type heat exchangers.