Table of Contents
The co-occurrence of the sulphides of zinc and iron is frequent; blende and pyrite, or marcasite, are found together in important ore bodies which are worked for the value of the contained zinc, and many lead deposits in their lower horizons carry zinc and iron sulphides. Galena may be separated in the wet way from both of the lighter sulphides, but the specific gravities of the latter are too similar to permit their separation from each other by any method depending upon specific gravity.
The presence of iron in zinc ores is very undesirable for metallurgical reasons connected with the reduction of zinc, and this fact, together with the similar specific gravities of the sulphides of these metals, gives rise to one of the most important fields of magnetic separation.
The middling products from mills treating galena-blende-pyrite ores frequently carry an important value in gold and silver locked up in the pyrite. The presence of any considerable amount of zinc in such middling renders it unsalable at the lead smelters, or involves the payment of a heavy penalty for each unit of zinc above a certain standard, usually 10 per cent., and their iron content renders them unsalable at the zinc smelters. If, however, the pyrite and blende are separated, two valuable products result.
Pyrite (FeS2, sp. gr. 4.8 to 5.2) is almost nonmagnetic; in some specimens it has been reported as diamagnetic, and in others as possessing a feeble paramagnetism; it is not attracted by the fields of the most powerful separators. On roasting it is readily transformed into the magnetic sulphide, and on the continuation of the roast, into a strongly magnetic oxide analogous to the mineral magnetite. Pyrite, in some specimens, on being heated at a quite low temperature for a minute or two, develops an iridescent film of magnetic sulphide, which imparts sufficient permeability to cause it to be attracted by fields of low intensity. The varying magnetic behavior of pyrite may be in some way connected with its several crystalline forms.
Marcasite (FeS2 sp. gr. 4.6 to 4.85) is similar to pyrite in its magnetic qualities.
Sphalerite or blende (ZnS, sp. gr. 3.9 to 4.2) varies in permeability according to the percentage of isomorphic iron and manganese sulphides contained by it. The pure sulphide of zinc, as represented in the light-straw colored varieties, is diamagnetic, while the highly ferriferous variety, called marmatite and “black jack,” in which the combined iron may reach 12 or 14 per cent., may be even ferromagnetic. Pure blende, or “rosin jack,” carries 67 per cent. zinc, while “marmatite” rarely carries over 51 or 52 per cent. zinc. Blende carrying as low as ½ per cent. isomorphous iron becomes appreciably magnetic upon roasting. Blende is being separated as a magnetic product at several mills in Colorado, in Europe and in Australia. The degree of magnetism appears to depend upon the ratio of the two sulphides contained, which varies from 3 parts ZnS to 1 of FeS2, to 5 parts ZnS to 1 of FeS2, and in any given ore the individual crystals of sphalerite may vary from the nonmagnetic straw-colored blende to strongly magnetic marmatite. A dark color is not necessarily indicative of high iron content.
Separation of Roasted Pyrite and Marcasite from Nonmagnetic Blende
As neither pyrite nor marcasite possesses sufficient permeability to be attracted by even the most intense magnetic fields, a preliminary roast is necessary before they may be separated from the blende. There are two methods for rendering iron sulphide magnetic: a slight roast with the formation of the magnetic sulphide, or a more complete roast with the formation of a magnetic oxide of iron.
The magnetic compounds of iron formed by roasting the sulphide are strongly magnetic and are attracted by fields of low intensity, but, as the quality of the separation made depends entirely upon the uniform magnetic quality of the material presented to the separators, the roasting is the most important step in the whole process. Almost any separator can make clean products when fed with properly roasted material, but no separator can do satisfactory work upon a poorly roasted feed.
Upon roasting pyrite or marcasite with access of air a portion of the sulphur is driven off as SO2 and the nonmagnetic FeS, (pyrite) is transformed, superficially at least, to Fe7S8 (analogous to pyrrhotite) which is strongly magnetic. This operation is a difficult one to control in most furnaces, however, as it is easy to oxidize some of the iron, making a product of uneven permeability.
If the sulphur is completely driven off by the roast Fe3O4 results, which is strongly magnetic. Should the roast be carried farther, another atom of oxygen is taken up by the iron and Fe2O3 results, which is quite feebly magnetic, being analogous to the mineral hematite. These two oxides of iron pass from one to the other, according as the atmosphere of the furnace is reducing or oxidizing. The artificial magnetite, the black oxide produced by the roast, loses its magnetism more readily than the natural magnetite, and is converted into the feebly magnetic red oxide. This may in turn be converted back to the black oxide by exposing it to a reducing atmosphere at the end of the roast.
Pyrite and marcasite begin to loose their sulphur and change over into the magnetic sulphide, and finally into the oxides, at a temperature of 370° C, and the roast must be conducted between this point and the ignition point of blende, which is about 600° C. Below 400 to 460 degrees the pyrite does not become thoroughly magnetic and the usual temperature employed is just below the ignition point of blende. If this temperature be slightly exceeded the only result is a superficial oxidation of the blende; a temperature of 620 degrees was attained without harmful results in some experiments carried out by Messrs. Hofman and Norton. Should this heat be maintained, however, a serious loss would result through the oxidation of the fine particles of blende.
In plants where the quantity of material treated is sufficient to make it feasible, the ore, or concentrate, should be sized before roasting. Roasting and magnetization take place from the surface inward, and in a mass of ore composed of coarse and fine particles the finer sizes will have been overroasted before the lumps have been affected to their centers. If a medium roast is given the mixture the larger lumps will have centers of unchanged pyrite, while the fine particles may have been converted into the nonmagnetic sesquioxide, and it is evident that a clean separation of such a product is out of the question. If a quick, light roast is carried out with a view to the formation of a film of magnetic sulphide on the surfaces of the particles, a fairly uniform product may result from the treatment of unsized material; the same is true when the roast is carried to the complete formation of the magnetic oxide in a reducing atmosphere. With large lump ore it is difficult to tell when the roast has penetrated to the centers of the lumps, and the process requires too much time. With very fine material the interstitial spaces are small, and, the material having a tendency to pack, the reducing gases reach all the particles with difficulty; also, very fine particles of blende may be converted into the oxide of zinc and pass out of the furnace with the gases, and dust chambers must be provided to save as much of this material as possible. While it may not be definitely stated, certain experimenters have given 8 mesh as the best size for blende-pyrite concentrate for good results in roasting.
If the roast be conducted with too free an access of air, the particles will be made up of concentric rings of different magnetic permeability, the surface will consist of a layer of nonmagnetic sesquioxide beneath which will be found a layer of black magnetic oxide, enclosing, perhaps, a core of unchanged sulphide, the division between the two being marked by a layer of the magnetic sulphide. Unless the roast has been carried too far the magnetic oxide will impart sufficient permeability to the whole particle to cause it to be taken up by the magnet, unless decrepitation breaks these layers apart, in which case each behaves according to its individual permeability, and nonmagnetic iron finds its way into the blende concentrate. Separation should be carried out upon ore as it comes from the furnace, cooled in such a manner as not to induce decrepitation, and no part of the separator feed should be crushed after roasting. Particles which have been fritted together should be recrushed, but also reroasted before separation.
Much of the concentrate roasted ranges in size from 1/16 to ¼ in., but after roasting, all but a small percentage of the iron in this concentrate will pass a 20-mesh screen. This is due to the breaking up of the particles under the influence of the roast. If a partially roasted particle of iron sulphide is broken, a network of fine black lines of the oxide will be seen, reaching perhaps the center of the particle, the spaces between them being composed of unchanged sulphide. This seems to indicate that the roast proceeds more rapidly along the boundaries of the crystals (forming an aggregate of the mineral) than through the individual crystals, causing these aggregates to split up. The tendency of pyrite and marcasite to decrepitate at a lower temperature than blende has been employed to separate these minerals, screening following the roast.
There are many types of roasting furnaces on the market which, with proper management, may be made to do efficient work. The principal requirement is that the admission of air shall be under complete control. Several forms of mechanical furnaces are in extensive use, and the old shaft furnaces may be made to do good work; for the finer sizes, hearth furnaces do good work. The time required for a good roast may be said to vary from ½ hour to 2 hours for fine concentrate, up to 3 or 3½ hours for coarse material, roasting for the magnetic oxide. The roasted pyrite or marcasite should be dark-brown in color, almost black; a decided reddish tinge indicates overroasting. The magnetic sulphide is black, and requires little time for its formation.
If there is tendency toward overroasting, the air inlets should be sealed up, and all entering the furnace should be made to pass through the fire box, where such is used. The addition of a little coke or hard coal may be resorted to at the end of the roast to re-convert to the black oxide any nonmagnetic red oxide which may have been formed. Subjecting the roasting ore to the action of reducing gases is successfully employed in Europe for this purpose.
It is probable that in many American plants whose output is not sufficient to warrant the installation of the more expensive types of furnace a small hearth would be advantageous for the re-treatment of the middling product from the separators.
The roast may be considered satisfactory when the separators make a recovery of from 85 to 90 per cent. of the total zinc in the raw concentrate, and yield a clean blende product carrying 1.5 to 2.5 per cent. iron due to pyrite. The iron content may, after the best work, reach a much higher figure, due to the presence of combined iron in the blende. The Joplin and Wisconsin ores usually do not carry to exceed ½ per cent. combined iron. The Leadville ores offer a more difficult problem, owing to the presence of blende of all degrees of permeability: an extraction of 75 to 85 per cent. in a product carrying 40 to 50 per cent. zinc is good work. Direct separation of these ores yields a lower grade product and a less extraction.
Separation of Magnetic Blende from Pyrite
Magnetic blende is of frequent occurrence at Leadville, Colorado, and other parts of the Rocky Mountain region, and is also found in important ore bodies in Europe and Australia. The Colorado ores of this type usually carry galena, sphalerite, and pyrite with subordinate amounts of pyrrhotite; the galena is usually argentiferous and the pyrite may or may not carry the precious metals. The sulphide of zinc in these ores varies from straw-colored blende to marmatite—in other words, from a nonmagnetic mineral to one possessing sufficient permeability to be removed magnetically in its raw state.
The determination of a process for the treatment of any ore carrying magnetic blende should be done by actual test on sufficiently large samples to indicate commercial results. If the blende is wholly or in large part magnetic, then a direct separation on high-intensity separators is in order. Individual particles of marmatite may show ferromagnetism, but the bulk of the mineral in an ore is usually less strongly magnetic, and a field of high intensity is necessary to obtain a satisfactory recovery. The value of the pyrite in gold and silver plays an important part in the determination of the process to be followed; if its value is negligible, then any loss of blende in the nonmagnetic tailing merely results in a decrease in the percentage recovery of the zinc; but if the pyrite is valuable for contained gold and silver, this nonmagnetic tailing should be kept below the zinc penalty limit set by the lead smelters to which this product is destined. In choosing between direct separation and separation after roasting, the higher value at the smelter of roasted pyrite over the raw sulphide should be considered.
At one plant treating marmatite ores, the nonmagnetic blende remaining in the pyrite tailing is removed by electrostatic separators. When there is much of this nonmagnetic blende in an ore, and the value of the pyrite in gold and silver is sufficient, the nonmagnetic tailing may be roasted, and the iron and blende recovered separately as clean products. With ores which require roasting, a preliminary treatment of the raw ore on magnetic separators to remove the strongly magnetic marmatite is advisable, as the actual passing of the ore over a separator represents but a very small proportion of the total cost of preparing the ore for magnetic treatment, and this magnetic blende would otherwise find its way into the roasted iron tailing.
At Kokomo, Colorado, the Kimberly-Wilfley Mines Co. is separating pyrite from galena and blende after roasting to the mag-
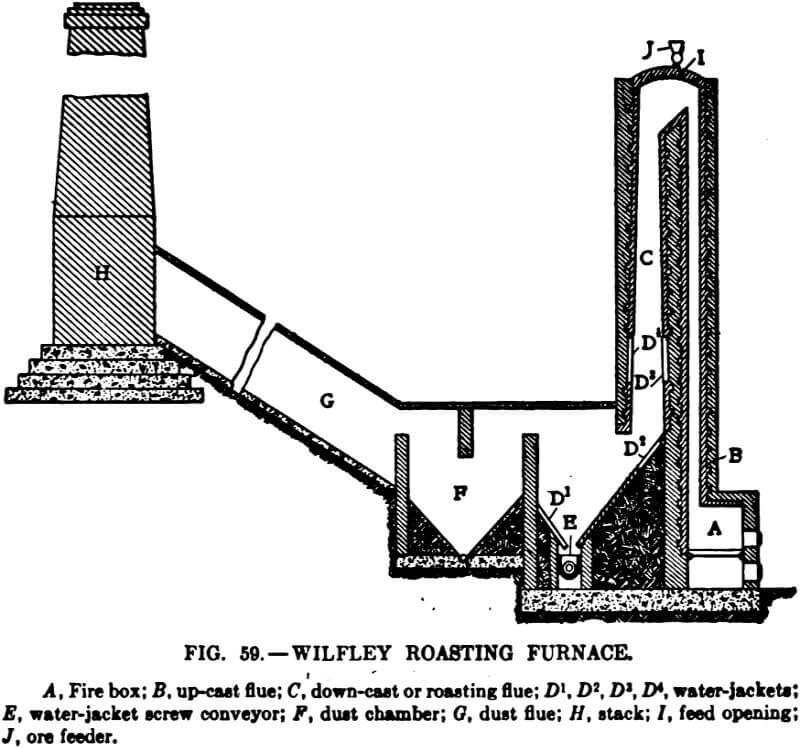
netic sulphide. The ore carries galena, slightly magnetic blende, and pyrite containing gold and silver values. The ore from the mine, after passing through a breaker, is crushed by 16 x 42-in. rolls to 3/8 in. and is then sized and gradually reduced to 14 mesh by three sets of 12 x 36-in. rolls. The crushed ore is transported by a conveyor belt to a Wilfley roasting furnace, a cross section of which is shown in the accompanying figure.
The ore fed at the top of the furnace falls upon a plate set at an angle of 50 degrees, thence through the down-cast flue, striking upon water-cooled plates, and finally into a water-jacketed screw conveyor which discharges it from the furnace. The roasting is carried out by the hot gases from the fire box together with the heat generated by the oxidizing pyrite upon individual particles, which are cooled to a certain extent before coming into contact with other particles, preventing fritting, and gives a uniform roast to the small and large particles alike. The ore is cooled sufficiently before passing out of the furnace to prevent a continuance of the oxidation when it comes into contact with the atmosphere. The dust
is collected in F and G, and is discharged from the furnace by a screw conveyor.
The fuel consumption is low, most of the heat necessary for the roasting being generated by the oxidation of the pyrite, the coal being used to regulate the temperature of the roast. It is said that during steady operation the temperature of the roasting flue does not vary more than 10 degrees, and a variation in the feed does not cause a variation of more than 100 degrees. The temperature is indicated by a pyrometer, the thermal couple being placed in the roasting flue above the water jackets.
The ore, as delivered from the furnace, is elevated and passed through a water-jacketed revolving cylindrical cooler, in which the temperature of the ore is reduced to 60° F., and sent to the separator bins. Ten Dings separators are employed to effect the separation of the roasted ore. The magnetic iron product from these separators falls upon a belt conveyor which delivers to shipping bins, whence it is shipped to the lead smelters. The nonmagnetic product falls upon another belt conveyor delivering to an elevator, is mixed with water and passed to two Richards classifiers, making four sizes; thence it is fed to eight Wilfley roughing tables. The pulp and middlings are sent to eight other Wilfley tables placed on the floor below and directly beneath the roughing tables. The tabling plant is operated in two units of eight tables each: one lower table receives all the zinc middlings, one all the lead middlings, one all the iron middlings not rendered magnetic in the roast, and one all the silica middlings from four of the roughing tables above. The overflow from the classifiers, the crosswash from the feed end of the Wilfley tables, and the dust from the furnace go to a Buckingham filter tank, which classifies and dewaters the fines and makes a thick pulp that is fed to a Wilfley table and to a Frue vanner fitted with egg-shell belt. It is stated that the roasting of the ore so changes the galena and blende that even the finest particles will not float, and renders easy an otherwise difficult separation.
The plant has a capacity of 250 tons per day.
At Denver, Colorado, the Colorado Zinc Co. is operating a plant of 100 tons daily capacity, equipped with a Wilfley furnace and Dings separators, with Wilfley tables for the separation of the nonmagnetic product. The ore is crushed dry to 16 mesh, passed through the furnace where from 10 to 15 per cent. sulphur is driven off, transforming the pyrite to the magnetic sulphide. After cooling in a water-jacketed cylindrical cooler, the ore is passed over 4 Dings separators which remove the iron, amounting to from 40 to 50 per cent. of the ore, while the nonmagnetic product is sent to the tables. The furnace is 8 x 8 ft. in section and 30 ft. high, and has a capacity of 100 tons per 24 hours. With ore carrying 25 per cent. iron as pyrite the furnace requires 1 ton of coal per day when operated at capacity. The separators deliver a finished iron product and a silica-zinc-lead middling which is sent, after sizing, to four Wilfley tables; these tables are operated to make finished zinc concentrate, while the lead-zinc middling and the silica-zinc middling are re-treated on three other tables. It is stated that, due to the action of the roast, the galena and blende do not, in the finest particles, float, and that the wash-water from the tables runs clear, while when the ore is tabled without roasting the wash water contains from 10 to 15 per cent. of lead-zinc slime.
At Galena, Illinois, the Joplin Separating Co. is operating a custom plant whose raw material is derived from the adjacent Wisconsin zinc field. Zinc-iron concentrate from mills not equipped with separating plants forms the greater part of the material treated; raw ores carrying zinc, iron, and lead sulphides are also purchased and, after water concentration, magnetically cleaned.
This plant is well managed and, although running on all grades and classes of material, probably represents the best practise of the district. The mill includes a roasting furnace of 40 tons capacity per 24 hours, three Cleveland-Knowles 12-in. belt separators, and a complete equipment of jigs, tables, etc., for the concentration of raw ores preliminary to roasting and magnetic separation.
The Wisconsin ores carry blende, galena and marcasite in a limestone gangue. The ore is easily crushed and yields the bulk of its component minerals when crushed to 4 mesh, although a finer comminution is carried out on the middling products from the jigs. The blende is of the variety known as “rosin jack,” and rarely carries to exceed ½ per cent. combined iron: some darker- colored blende is produced in the district, but none that came under the writer’s observation was sufficiently magnetic to be affected by the low-intensity magnetic fields employed in the separation of these ores. The purity of the ores is well illustrated by the fact that 60 per cent. concentrate is the standard grade from which prices are figured, and iron in excess of 2 per cent. is penalized at the rate of $1 per unit.
In this mill the blende-marcasite concentrate is delivered to the feed hopper above the roasting furnace by a 6-in. bucket elevator. The feed hopper is 36 ins. square and slopes from two sides to a point. The feeder, of the stirrup type, delivers into a sheet-iron spout which extends well into the neck of the furnace.
The furnace is of the revolving-cylinder type, built by the Galena Iron Works, and is 32 ft. long by 5 ft. in diameter. It is built of boiler plate and lined with fire brick. This shell is fitted with two tires which rest upon two sets of rollers, the distance apart of which may be adjusted to give any desired inclination from the horizontal to the axis of the cylinder, so accelerating or retarding the passage of the ore through it. Revolution is imparted to the cylinder by gearing. At either end the furnace is narrowed by fire-brick walls to 2 ft. 6 ins. for connection with the fire box at the discharge end, and with the dust chamber, which also serves as foundation for the stack, at the feed end. These connections are made through cast-iron necks projecting into the openings at each end of the cylinder. The fire box is fitted with a grate 4×5 ft. in area, which burns about two tons of soft coal in 24 hours. The roasted ore is discharged through the annular opening between the projecting neck of the fire box and the end of the cylinder. A fire-brick wall reaching the horizontal diameter of the fire-box neck causes the hot gases to impinge on the roof of the cylinder and not to strike the hot ore.
A 24-in. rotary blower is mounted alongside the furnace and suitably connected to furnish air under pressure beneath the grate. This is useful in raising the temperature of the charge quickly should it fall below normal and an imperfectly roasted product be likely to result.
The roasted ore discharged from the furnace falls upon a cast-iron plate, and is conveyed to one side by scrapers mounted upon a traveling chain, and falls into wheelbarrows.
The furnace makes two revolutions in three minutes, the ore remaining in it for approximately two and one half hours.
The hot ore is wheeled to a cooling floor 26 ft. wide by 60 ft. long; here it is spread a few inches deep and allowed to remain 12 hours. The ore was formerly cooled by means of a spray of water, but this gave rise to an excessive amount of fines, produced by the sudden cooling, so that it was abandoned in favor of the cooling floor, in spite of the increased cost of handling entailed.
The cooled roasted material is raised to the top of the mill by a bucket elevator and fed into a trommel 36 ins. in diameter. This. trommel is fitted with two screens, each delivering its undersize to a separate bin, while the oversize from both is crushed in tight rolls and returned. The first screen has 3-32-in. and the second 3/16-in. round punched holes.
The roasted concentrate passing 3/32-in. is treated on separator No. 1, which carries ¾ ampere on the first magnet and 6.5 amperes on the second, making a clean iron tailing product and a clean nonmagnetic zinc product. The material between 3/32 and 3/16 in. is treated on separator No. 2, which carries the same current, re-
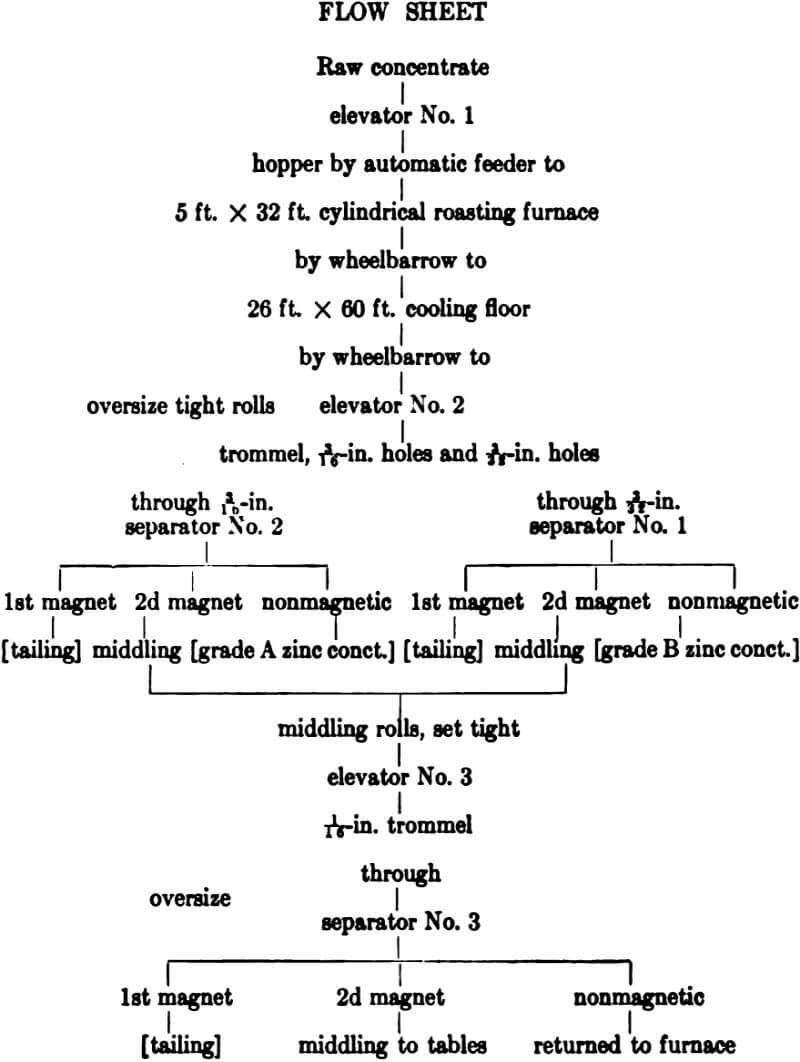
spectively, on the two magnets. The middling product from both these machines is crushed in tight rolls to 1/16 in., and fed to separator No. 3, which also makes three products. The tailing from the first magnet is discarded while the middling from the second magnet is sent to the tables in the concentration mill. The non magnetic product from this separator carries most of its iron as unchanged pyrite, liberated by the recrushing of the middling from separators Nos. 1 and 2, and is, therefore, fed back to the roasting furnace.
The speeds of the separator belts are adjusted to suit each class of material treated.
The magnetic tailing is run to waste through a launder in which a stream of water is kept flowing, and the cleaned blende is delivered to shipping bins through chutes.
The finished product is sold under two grades: grade “A” is the product from the separator treating the coarser size and grade “B” from that treating the finer size; it has been found here that the coarser the size of the concentrate the higher the grade. The average selling assays on 33 car loads gave grade A 60.49 per cent. zinc and 2.11 per cent. iron, and grade B 57.07 per cent. zinc and 2.19 per cent. iron. The total amount shipped was in the proportion of two cars of B to each car of A.
The raw concentrate purchased by this mill carries from 11 to 33 per cent. zinc. The tailing produced averages below 5 per cent., which figure is said to be never exceeded. The efficiency of the plant is given as 85 per cent. of the zinc in the raw concentrate.
The mill is equipped with a complete series of rheostats to control the currents on all magnets from the central switchboard.
At Hazel Green, Wisconsin, the Kennedy Mining Co. employs a Cleveland-Knowles separator to clean roasted blende-marcasite concentrate. The concentration mill treats 100 tons of ore daily for a production of 25 tons of concentrate, carrying from 41 to 42 per cent. zinc. From the mill the concentrate is delivered to the roaster building by a self-dumping skip and drops into the boot of a bucket elevator, which delivers to the furnace feed hopper. The furnace is of the cylindrical type usual in the Wisconsin district and is 28 ft. long and 5 ft. in diameter. It is set at an inclination of 4 ins. in 28 ft. and makes two revolutions in 3 minutes. The ore remains in the furnace from 3½ to 4 hours. The cylinder is lined with 8-in. fire brick between which are set at intervals projections of refractory material in the form of equilateral triangles with an altitude of 3 ins., which serve to lift the ore by the revolution of the furnace and allow it to fall through the hot gases from the fire box. About two tons of soft coal are burned in 24 hours. The roasted concentrate falls from the furnace into a paddle conveyor, where it is sprayed with water, which is evaporated immediately by the hot material, which it serves to cool. This conveyor delivers to a bucket elevator delivering into a trommel above the separator bins. The roasted concentrate is here sized into two products, through 1/16 in. and between 1/16 in. and ¼ in.; these two sizes are treated at different times by the separator and the oversize is recrushed. The first magnet of the separator, which is a 21-in. Cleveland-Knowles machine, takes 1.5 amperes and the second magnet 3.5 amperes. The separator belt travels at a speed of about 175 ft. per minute. The magnets revolve at 75 R.P.M. at a height of 1 in. above the belt. The separator treats from 20 to 22 tons of roasted concentrate in 24 hours, which indicates a burden of from 1.25 to 1.5 lbs. of material on the belt at any one time; this quantity is carried as an even layer one particle deep. The first magnet takes out an iron-tailing product which is run to waste in a launder; the second magnet removes a middling product which is fed to the middling rolls in the concentration mill—a procedure of doubtful economy. The nonmagnetic material remaining on the belt is almost clean blende, with a little limestone and galena which were not eliminated in the concentration mill, and not being magnetic, these are concentrated by the separation in the same proportion as the blende. The cleaned zinc product amounts to about 16.5 tons in 24 hours and assays 60 per cent. zinc with 2 per cent. iron. The tailing carries about 5 per cent. zinc.
At Platteville, Wisconsin, the Enterprise Mining Co. is separating about 15 tons of blende-marcasite concentrate daily. The concentrate is delivered from the mill to the roaster bin by a skip running on an incline. The Galena cylindrical roasting furnace is employed, the concentrate remaining in the furnace three hours. The roasted concentrate is sized in a trommel into three products: through 1/8 in., between 1/8 in. and ¼ in., and oversize. The first two are fed to a 21-in. Cleveland-Knowles separator separately, the bin being provided with a partition to keep them apart; the ¼-in. oversize is crushed and refed to the furnace. The separator carries 3 amperes on the first magnet and 5 amperes on the second. The tailing from the first magnet is run to waste in a wet launder and the middling from the second magnet sent to the jigs in the concentrating mill; a falling off in the average grade of the cleaned zinc product from 61 to 59 per cent. is said to have resulted from so treating the middling. The cleaned zinc concentrate carries from 58 to 62 per cent. zinc and averages about 2 per cent. iron. The raw concentrate fed to the furnace carries from 40 to 45 per cent. zinc. The tailing from the separator is said to carry 4.5 per cent. zinc.
The Empire Mining Co., of Platteville, is operating a plant similarly equipped. From 18 to 20 tons of raw blende-marcasite concentrate is treated daily for a recovery of from 10 to 15 tons of cleaned zinc. The cleaned zinc concentrate carries from 60 to 63 per cent. zinc and from 1 to 3 per cent. iron. Hocking Valley Coal is burned by the furnace, costing $5 per ton delivered; the furnace uses from 1500 to 2000 lbs. in 24 hours.
At Mineral Point, Wisconsin, the Mineral Point Zinc Co. operates a custom magnetic-separating plant in conjunction with a zinc-reduction works. The raw material is gathered from all parts of the district and consists chiefly of the products of water concentration ranging in size from ¼ in. downward; crude ore is occasionally treated. The concentrate fed to the furnace ranges from 28 to 34 per cent. zinc.
The roasting plant is equipped with two cylindrical furnaces of the Galena type, only one of which is at present in use. This is 20 ft. long by 5 ft. in diameter. The lining of this furnace is of 8-in. special-arch fire brick in which are set four rows of 10-in. brick spaced 90 degrees apart; these elongated brick serve to lift the ore by revolution of the furnace and allow it to fall through the hot gases; the substitution of 14-in. brick in place of the 10-in. is contemplated. This furnace is equipped with a dust chamber 5 ft. 4 in. wide by 14 ft. in length, is divided horizontally by a plate of heavy sheet iron. The gases from the furnace pass into the lower compartment of the dust chamber, along beneath the plate to the farther end, where a 2-ft. space is left between the end of the plate and the wall of the dust chamber, and thence back over their course, but above the plate, to the stack, which is 30 ins. in diameter and 50 ft. high. The dust is removed through three doors, two on the upper level, and one on the lower, each 12 ins. wide by 2 ft. high. The fire-box grate is 4 x 5 ft. in area, and burns from 1.5 to 2 tons of soft coal, costing $3 per ton, daily. The cylinder makes one revolution in 50 seconds, the ore remaining in the furnace from 3 to 3.5 hours. The temperature of the roast is just sufficient to start a slight fritting at the discharge neck of the furnace; that this action is incipient is shown by the fact that little trouble is experienced from overroasting or from particles of zinc and iron which have been cemented together.
The slight deposit which forms at the discharge neck is removed from time to time with a long chisel. Paddle-and-chain conveyors are used to convey the roasted ore from the furnace, and a small stream of water is sprayed upon the hot material to lay the dust and assist in cooling it; the water used is regulated so that it may be completely evaporated by the heat of the ore before it

reaches the separator bins. The furnace treats about twenty tons of raw concentrate in 24 hours.
The roasted concentrate is separated on a Cleveland-Knowles 21-in. separator and upon a Dings separator, set up side by side and working on the same material. Either machine is capable of treating the output from one surface. The Cleveland-Knowles carries 3 amperes on the first magnet and 6 amperes on the second: the Dings separator carries 3 amperes on either magnet. The roasted concentrate is sized before separation into two products, between 1/8 and ¼ in., and through 1/8 in. The concentrate averages 59 per cent. zinc and 2.5 per cent. iron; the middling amounts to
from 7 to 10 per cent. of the feed and carries 17 per cent. zinc; it is stacked awaiting a process. The tailing product averages from 2 to 2.25 per cent. zinc. An efficiency of from 85 to 86 per cent. of the zinc in the raw concentrate recovered in the cleaned zinc product is claimed for the plant.
Power for the furnace and separators is supplied by a 15 H.P. motor.
The mill of the Tripoli Mining Co., situate three miles northwest of Mineral Point, is separating blende-marcasite concentrate on Dings separators. The concentrate from the mill is trammed to the roaster building, some 200 ft. away, in mine cars, and dumped at the foot of a bucket elevator delivering to the furnace feed hopper. The furnace, of the cylindrical type, makes one revolution in 1 minute and 40 seconds and roasts from 18 to 20 tons of concentrate in 24 hours. The roasted ore from the furnace falls into a screw conveyor 15 ins. in diameter and making 24 R.P.M., and from this into a chain conveyor running at a speed of 2 ft. per second; there are 23 ft. of screw conveyor and 15 ft. of chain conveyor, and in this distance the roasted material is sufficiently cooled to be fed into the separator bins. A noticeable grinding action is set up in these conveyors.
The roasted concentrate is separated on a Dings separator. The raw concentrate carries from 30 to 35 per cent. zinc, and the cleaned zinc product from the separator from 57 to 59 per cent. zinc with from 2.5 to 5 per cent. iron.
A motor taking 44 amperes at 125 volts drives the furnace, conveyors, elevators, separator, etc.
At Joplin, Missouri, the Joplin Separating Co. is operating a custom separating plant on blende concentrate. The iron content of the raw concentrate averages 15 per cent., which is reduced to an average of 1.06 per cent. in the cleaned zinc product. The roasting is done in kilns, and the separation on two Cleveland-Knowles separators.
At Kaslo, British Columbia, the Kootenay Ore Works operates a custom works on concentrate carrying galena, blende and pyrite carrying from 15 to 20 per cent. iron and rarely exceeding 37 per cent. zinc. The ore is delivered from railroad cars to bins at the top of the mill and after passing a sampler is fed to a White-Howell roasting furnace. The roasted ore is cooled in a revolving cylinder through which a current of cold air is passed. After cooling, the ore is crushed to pass 20 mesh and classified into eight sizes, and the iron removed on four Dings separators. The final zinc product carries from 7 to 8 per cent. iron.
At Huanchaca, Bolivia, ” Stern” type separators are employed to separate blende-pyrite ores after roasting. There are five of these machines in operation; three of them treat original ore, and two others are used to clean the concentrates from the first machines. The capacity of the plant is 2 metric tons per hour.
The ore from the mine is crushed to 30 mm. in a breaker,
which is followed by rolls, further reducing the ore to pass a 4-mm. screen.
From the rolls the ore is lifted by a bucket elevator and fed to a roasting furnace of the revolving-cylinder type. The roast drives off sulphur from the pyrite, forming the magnetic oxide of iron. The roasted ore is let fall into a conveyor, where it is cooled by the action of a stream of cold water, and thence is carried to a trommel with 4-mm. screens. The oversize, due to swelling in the roast, is returned to the rolls, while the material which passes the screen is delivered to feed hoppers supplying the separators. The ore at this point is mixed with the proper quantity of water in a specially constructed funnel mixer, and next fed to the roughing separators, three of the Stern-type wet machines.
The nonmagnetic blende is caught in a trough beneath the separators and after settling is drawn off, constituting a finished product. The magnetic roasted iron is delivered to a tube mill and reduced to 2 mm., and from thence is elevated by a centrifugal pump to settling tanks from which it is tapped to the cleaning separators, two in number, of the same type as the machines working on raw ore.
The final results of the magnetic separation are a concentrate carrying 50.81 per cent. zinc and tailing averaging 3.98 per cent. zinc; the feed averages 30.81 per cent. and the extraction in terms of total zinc in the feed is 98 per cent. On a somewhat lower grade of ore the following results are obtained: concentrate, 40.10 per cent. zinc; tailing 4.80 per cent. zinc; the feed averaging 24.22 per cent. and the extraction 91.3 per cent.
At Pueblo, Colorado, the Mechernich separator is employed by the United States Zinc Co. for the separation of blende from pyrites, and the other mixed sulphides. The ore is given a roast preliminary to separation.
At Ravalo, Sweden, Herbele separators are employed to separate zinc-iron-lead ores.
At Munsterbusch, near Stolberg, Germany, the Aktien-Gesellschaft fur Bergbau-Blei and Zinkfabrikation is separating roasted blende-pyrite concentrate on an 80-cm. Mechernich separator.
At Hamborn, Germany, the Aktien-Gesellschaft fur Zink-Industrie is operating a plant equipped with Humboldt-Wetherill separators on blende-pyrite concentrate.
At Lipine, Upper Silesia, Germany, the Schlesischen Aktien- Gesellschaft is separating roasted blende-pyrite concentrate on Mechernich separators. The raw material carries from 25 to 26 per cent. zinc, which, after a slight roast, is passed over a Mechernich double-pole separator. This machine treats an average of 1.5 metric tons per hour from which is produced 0.9 ton of zinc product assaying about 40 per cent. and 0.6 ton iron product assaying 15 per cent. zinc, indicating an extraction of 80 per cent.
At Carlshof, Germany, Henckel von Donnersmark is operating a plant of 20 metric tons capacity in 10 hours on roasted blende-pyrite concentrate, employing the Humboldt separator.
At Peyrebrune, Germany, the Peyrebrune Co. is operating a separating plant equipped with Humboldt separators on blende-pyrite concentrate; the capacity of the plant is 8 metric tons in 10 hours.
At Kattowitz, Germany, there is a magnetic separation plant equipped with twelve Stern wet-type separators treating roasted blende-pyrite ore and concentrate. The capacity of the plant is from 5 to 7.5 metric tons per hour.
At Torrelavega, Santander, Spain, the Real Compania Asturiana de Minas is operating a magnetic separation plant on blende-pyrite concentrate.
Separation of Magnetic Blende from Pyrite
At The Yak Mill, Leadville, Colorado, International separators are employed to separate magnetic blende. The ores treated at this mill carry blende, pyrite and pyrrhotite and, as nearly as can be learned, carry from 20 to 30 per cent. zinc. The ore is treated raw, the blende being recovered as a magnetic product. There are eighteen of these machines installed here, four of which receive the initial ore; the others treat the products of the original machines. The daily capacity of the mill is from 200 to 250 tons, making the amount handled by the primary separators upward of 50 tons each. The capacity of one of these machines on 20 per cent. ore is stated at 2.5 tons per hour, and 3 tons per hour on 30 per cent. ore. The ore is reduced dry to pass a 0.043-in. screen aperture. The eighteen machines use 64 amperes at 250 volts for excitation, or less than 1 kwt. per machine. One horse power is ample for the mechanical operation of the separator. No data as to the results obtained are available. Experiments are now being carried on with the Cleveland-Knowles separator, the ore being given a preliminary roast.
At Denver, Colorado, the Colorado Zinc Co. is treating magnetic blende ores on Wetherill-Rowand separators and Blake-Morscher electrostatic separators. The ores treated come from Georgetown, Black Hawk, Breckinridge, and Leadville, and carry magnetic and nonmagnetic blende, galena, pyrite, and a little pyrrhotite. The ore varies in size down to classifier products, and carries from 22 to 30 per cent. zinc.
The plant is equipped with three Type E Wetherill-Rowand separators and three double Blake-Morscher electrostatic separators, together with Wilfley tables, crushers, rolls, etc., for the preliminary concentration of such raw ores as are purchased. At the time of the writer’s visit the material treated by the separators was the middling product from the Wilfley tables, crushed to 30 mesh before concentration, and carrying from 20 to 25 per cent. zinc with 25 to 28 per cent. iron as pyrite.
The middling from the tables is raised to a hopper by a skip, and fed into the upper end of a cylindrical drier revolving on an inclined axis, and from this is transferred to the separator bins, being cooled on the way.
The separators are standard size, 18-in. belt with six separating zones, operated at the rate of 17 tons of feed per machine per 24 hours, which appeared to be somewhat above their capacity, which is said to average 700 to 1000 lbs. per hour, at which rate each machine requires 7.5 H.P. for excitation and operation. The belt speeds for this tonnage were: conveyor belt, 45 ft. per minute; take-off belts, about 7 ft. per second; feeder, 6 R.P.M. The current employed on the several magnets could not be ascertained. The separators make four products, as follows:
- From the first pole of the first magnet, carrying least current, a rather strongly magnetic product consisting of pyrrhotite, small particles of pyrite which have been rendered magnetic in the drying furnace, etc. This material forms tufts or bunches which do not lose their magnetism for some seconds after leaving the magnets; it carries from 7 to 11 per cent. zinc, and is trammed to the waste dump.
- From the second pole of the first magnet, a light-colored product carrying from 12 to 15 per cent. zinc; it is repassed.
- From the four poles of the second and third magnets, finished zinc concentrate carrying from 41 to 43 per cent. zinc and 10 to 12 per cent. iron; the iron in this product is mostly in combination with the blende, but a small percentage is due to pyrite.
- Discharge from the conveyor belt, consisting of pyrite and nonmagnetic blende, and carrying from 10 to 14 per cent. zinc; it is sent to the electrostatic separators.
The zinc content of the products from the several poles increases directly with the current employed on the magnets; is lowest from the first magnet, which receives the least current and highest from the last, which receives the strongest current, and which apparently removes a greater quantity of product than any of the others.
The grade of the tailing product depends almost entirely upon the condition of the zinc in the feed; the more nonmagnetic zinc there is in the feed the higher the zinc content of this product. The tailing also carries a small amount of feebly magnetic blende, which would be taken up by the magnets were the capacity of the separators cut down and the speed of belt travel reduced.
At Canyon City, Colorado, the Empire Zinc Co. is operating a plant equipped with ten Wetherill-Rowand separators and one Wetherill Type F separator, on magnetic-blende ores.
At Ille et Vilaine, France, the La Touche Mining Co. is operating a plant of 12 metric tons capacity in 10 hours on magnetic blende ores. The Humboldt-Wetherill separator is employed.
The Societe des Mines de Balia Karaidin, Constantinople, Turkey, is operating a plant of 4 metric tons hourly capacity on unroasted blende-pyrite ores. The Humboldt-Wetherill separator is employed.
At Minaca, Chihuahua, Mexico, the Calera Mining Co. is operating Wetherill-Rowand separators for the recovery of blende from the tailing from an ore carrying galena, blende, and pyrite in a garnet gangue, which is concentrated on Sutton-Steele pneumatic tables. The concentrate obtained carries from 40 to 45 per cent, zinc.
