Table of Contents
To remain competitive, the domestic metals industry must not only make advances in mining methods, but also in ore processing. One processing advancement under investigation is the development of nonconventional solvent extraction (SX) stripping techniques such as hydrogen, hydrolytic, and precipitation stripping.
Solvent extraction selectively removes a metal from an aqueous solution into a solvent or organic phase containing an active chemical group inherently attractive to the particular metal ions. Conventional SX stripping removes the metal loaded in the organic by contacting it with a small volume of a concentrated aqueous eluant. The organic extractant is regenerated to its acid or uncomplexed form and the SX loaded metal is exchanged into a concentrated strip liquor. The resulting strip liquor is then treated by a separate operation such as electrowinning or precipitation to recover the metal. By replacing conventional aqueous stripping with hydrogen or hydrolytic stripping techniques, SX technology is streamlined by combining organic stripping and metal recovery operations into a single process step. A conceptual diagram of this operation is shown in Figure 1.
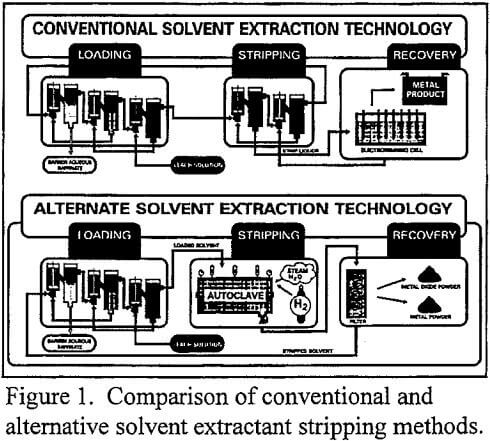
In hydrogen stripping, metal-loaded organic solvent is reacted with hydrogen gas in an autoclave at temperatures and pressures sufficient to reduce the loaded metal ions to the metallic state. The metal precipitates as a powder while the hydrogen regenerates the organic acid. The following equation illustrates the overall reaction for a divalent metal (M):
![]()
where R represents the organic-acid radical. This reaction is analogous to the hydrogen reduction of metals from aqueous solutions. Thermodynamics and solvent extractant selectivity limits the metals responsive to this reduction from aqueous or organic solutions to precious metals and the more noble base metals.
Hydrolytic stripping is a second alternative method to recover metal values from loaded solvent extractants directly as a solid powder product. The reaction is similar to hydrogen stripping except water, present as steam pressure inside the autoclave, hydrolyzes the bound metal into a metallic oxide, hydroxide, or oxy-hydroxide complex while restoring the extractant to its active hydrogen loaded form for metal reloading. The reaction has the added thermodynamic energy of the oxy-hydroxide compound formation to make up for the lower reducing potential of water substituting for hydrogen as the reductant. Equation 2 portrays the reaction for hydrolytic stripping a divalent metal.
![]()
A third alternative stripping technique is precipitation stripping, which involves boiling the loaded organic extractant with a dilute mineral acid strip solution. Hydrolysis of the bound metal from the ligands or organic complexes is driven to completion, consuming only water. This method is explained in more detail by Doyle (1992).
Initially demonstrated using heptanoic acid by Chalk and Halpern (1959), Burkin (1969, 1971) pioneered the application of pressure hydrogen reduction for the recovery of metal powders from commercial solvent-extraction reagents. Burkin’s screening tests indicated that, of the various extractants tested, Versatic Acid and di, 2-ethylhexyl phosphoric acid (DEHPA) were the most suitable. Comprehensive investigations and summaries of hydrogen stripping by Demopoulos (1981, 1985) and hydrolytic stripping by Thorsen (1979), Monhemius (1984), and Doyle (1985) have been previously published and will not be detailed in this document.
Monhemius (1984) effectively summarized the strategic advantages afforded by these alternative stripping methods: “The selective isolation of the metal ion in the solvent phase, as the extracted metal complex, offers a unique situation that can be exploited to produce high purity metal or metal oxide products. In these systems only metal cations are extracted; consequently, it is possible to produce organic phases containing only the ions of interest, with the complete absence of other inorganic ions. This situation is impossible to achieve in an aqueous system, since even a highly purified aqueous solution of a metal salt will contain, in addition to the cation of interest, accompanying anions plus hydronium and hydroxyl ions derived from the water.”
Extractants proven thermally stable in hydrogen stripping include Kelex-100 (7-[4 ethyl, 1 methyloctyl] quinoline) (Demopoulos, 1981), DEHPA (Burkin, 1971), LIX 65N (aryl hydroxyoxime) (Navarro, 1987), Butex (dibutoxy diethyl ether) (Li, 1984), and Versatic acid (tertiary carboxylic acid) (Thorsen, 1979)’. Only Versatic acid has been documented as compatible for hydrolytic stripping.
All previous investigations on the alternative solvent extraction stripping technology (ASXST) methods mentioned have focussed on synthetic solutions made from single or multiple reagent salts. This investigation reports the performance of ASXST using commercially available solvent extractants and complex solutions derived from actual ore, scrap recycling, and waste material. Three diverse materials were selected for investigation of ASXST to illustrate the wide range of potential applications, and because they were under investigation by several unrelated USBM projects to resolve metal recovery problems. Specifically, the materials were: 1) copper ore from Magma, AZ; 2) nickel metal-hydride (AB5-alloy) advanced battery scrap; and 3) Fansteel Ta-Nb processing waste.
Copper comprises 16% of the United States’ total dollar value for metal mining production (USBM, 1994). Leaching low grade or inaccessible ore deposits and processing by solvent extraction and electrowinning (SX/EW) provides an expanding portion of this program. Solution recovery of copper is envisioned to expand with the development of in situ mining methods at sites such as the Casagrande Joint Venture between the USBM, ASARCO, and Freeport-McMoran. Investigations by the USBM into copper recovery from in situ leach liquors have indicated that leach solution acidities much higher than those used in conventional dump and heap leaching may be necessary with in situ operations (Isaacson, 1991). Although these liquors may be too acidic for conventional SX/EW processing, copper extraction using the acid-tolerant Kelex-100 coupled with pressure hydrogen stripping appears to be well suited for processing these high-acid leach liquors. The accompanying corrosion and acid mist hazards would be minimized in the surface facilities by substitution of hydrogen pressure stripping for high-acid strip liquors.
AB5-alloy advanced batteries is one of the promising power sources under investigation for electric powered cars, and contains significant amounts of nickel, cobalt, iron, and rare-earth metals. In order to recover these valuable metals, the iron dissolved in the leach solution must be removed to prevent interference or contamination in subsequent processing. Iron hydroxide or jarosite precipitation produces voluminous, low grade iron residues which are difficult to filter, handle, dispose, store, or recycle, and can consume large amounts of reagents. The ability to recycle all components of automobiles at the end of their service life to minimize costs, waste, and pollution has become a key issue for the automotive industry, environmentalists, metal producers, and consumers in general. This research has become high profile in the United States’ efforts to compete and advance in high technology development (Illman, 1994). Application of SX coupled with hydrolytic precipitation directly from the loaded organic could produce a higher grade material than conventional precipitation with only the addition of water and heat.
Minimization of waste volume and losses of valuable metals are strong concerns in the disposal of metal processing wastes and will continue to grow as disposal sites are depleted. Zirconium metal, indispensable in nuclear reactor fuel containment, and high temperature and corrosive tolerant structures, can be recovered as a byproduct from tantalum-niobium processing residues containing high levels of refractory metals and radioactive materials. Zr metal is used to contain uranium fuel rods in nuclear reactors because it is transparent to the neutron radiation, while Hf is used to shield reactors because of its large neutron absorption cross section. Both metals exhibit excellent corrosion and radiation resistance in these applications. Thorium, uranium, scandium, hafnium, and thallium in addition to the tantalum and niobium must be separated from the zirconium for it to be useful. Both Zr and Hf are valuable metals, but the most critical application of Zr metal requires minimal levels of Hf, and vice versa. Separation is accomplished in a thiocyanate/methyl isobutyl ketone system, but the recovery of these metals prior to their purification could be enhanced by modifier selection or oxalic precipitation stripping.
Experimental Materials Equipment and Procedures
The copper chelating extractant Kelex-100 was selected to examine copper recovery from actual ore leach solutions. SX/EW technology application to process and recover copper from dilute sulfuric acid leach liquors is expanding and Kelex has the thermal stability necessary for hydrogen stripping as noted by Demopoulos (1981) in his investigation. Kelex-100, manufactured by Sherex/Witco (batch No. 142-72) extracts metals with affinity in the general order: Au>Pd>Pt>Cu>Pb>Ni>Co>Fe.
DEHPA produced by Albright and Wilson, Inc., was examined for iron extraction from leach solutions of battery scrap, and zirconium extraction from tantalum-niobium processing waste residue leach solution. DEHPA is used for iron removal and extraction of other metals in hydrometallurgical processes throughout the world.
Two modifiers used to enhance phase separation and stability in solvent extraction organics, but of widely contrasting compositions, were tested in the copper and zirconium recovery investigations. Nonylphenol (1- hydroxy, 4-nonylbenzene), an aromatic modifier, and the aliphatic alcohol decanol, both produced by Kodak, were tested at 10 volume percent (v/o) level in the Kelex organic and at 15 v/o for zirconium recovery in DEHPA. Decanol at the 10 v/o level was employed in one of the organics examined for AB5 scrap treatment. Phillips Orfom SX-12, which contains 22% to 23% aromatics, was the diluent selected to make up the balance volume of the organics. Exxon Exxsol D-80 (formerly Escaid 110) which contains less than 1% aromatics, was also evaluated for effects in ASXST application as a diluent in some of the copper testwork. Solvent extractants and their compositions employed in this investigation are listed in Table 1.
All batch and continuous pressure stripping experiments were performed in a PDC Machines, Inc. (Pressure Dynamic Consultants) 2-L autoclave constructed of 316 stainless steel. To comply with the safety requirements for operating with hydrogen gas under pressure, all equipment in the autoclave room were required to be explosion-proof per Class 1, division 2, group B hazardous location specifications. The autoclave mixer was magnetically coupled to an air drive motor, with a magnetic pickup on the shaft to supply a digital tachometer with the mixer speed. Heating was supplied by an external mica band type heater with a gas tap on the heater chamber to allow an inert gas purge, and regulated by a Red Lion CAL 9900 temperature controller with proportional, integral, and derivative control loops. Temperature was measured using a type J-thermocouple (iron-constantan) with an ungrounded junction isolated inside a thermowell. A differential pressure switch inside an explosion-proof box installed on the heater purge line would cut all power to the heater if the pressure dropped below the setpoint (3.45 kPa) to prevent an explosion if hydrogen gas had leaked into the room. A pyrex liner was used in the autoclave chamber in all batch work for containment and ease in cleanup.
Purified copper metal electrolytic dust from Fisher Scientific, screened at 75µ (200 mesh) with a mean diameter of 17.7µ was used for copper seed in the copper recovery investigation. Low sulfur iron powder distributed by Laboratory Equipment Co. (Leco) was used for seed in some of the hydrolytic iron precipitation tests.
Leachate containing 22 g/L Cu and 4.4 g/L Fe used in the cycled organic degradation tests was produced by contacting 100 g/L H2SO4 solution with minus 6-mesh, plus 10-mesh copper ore from Magma Copper, Arizona placed in three 1.8 m high by 20 cm (i.d.) diameter acrylic columns connected in series to the leach pump. The second batch of leachate was produced from minus 6.3- mm, plus 6- mesh ore leached with 50 g/L H2SO4 in the same leach columns. This solution was evaporated, then spiked with technical grade CuSO4·5H2O (Fisher Scientific) to produce the 39 g/L Cu leachate solution used for the seed addition test series and comparison to the cycled organic tests to examine Cu concentration effects using hydrogen stripping. The pH in both solutions was adjusted to 3.0 ±0.1 with H2SO4 and NaOH. Compositions of the solvent extraction aqueous feed solutions used in this work are displayed in Table 2.
Cu, Fe, Ni, and Co analyses were performed by atomic absorption (AA) using a Perkin Elmer Model 2100 Flame Absorption Analyzer. Organic samples were diluted 5/1 with isopropyl alcohol to perform Cu and Fe determinations. Zr, Ti, Ta, Nb, Ce, La, Mg, Ca, and P determinations were performed with a Jarrell Ash model 975 Atom Comp inductively coupled plasma (ICP) spectrophotometer. Sc and Hf determinations were performed by nuclear activation analysis. Samples were irradiated in a small nuclear reactor at the University of Utah, then measurement of gamma emissions were performed at the USBM using a high purity germanium gamma spectrometer. Carbon, hydrogen, nitrogen, and sulfur content in copper products were determined with a LECO CHNS-932, and oxygen content was analyzed with a LECO CS-144.
General SX and stripping procedures
Organic was contacted with the metal loaded leach solution for 5 min at an aqueous:organic (a:o) ratio of 1:1. After the organic and raffinate were separated using a separatory funnel, the organic was rinsed with deionized (d.i.) water at an a:o of 1:1 for 5 min, separated from the rinse water, and then filtered through Whatman PS-1 phase separation paper to remove entrained water and acid. In some of the tests the mixture was stirred with a glass rod in the separatory funnel to speed phase disengagement when significant amounts of emulsion had formed. Loaded organic was placed into a pyrex cylinder along with the designated amount of seed material (if used) and loaded into the autoclave within 24 hr of loading to minimize crosslinking interferences with stripping. Nitrogen gas flowed through the heater compartment purge line during autoclave heatup and operation. The stripped organic, or stripped organic and strip solution were filtered using Whitman No. 42 filter paper cones (approximately 2.5µmopening) after removal from the autoclave.
Copper hydrogen pressure-stripping: About 1 L of loaded organic and the seed metal were placed in the autoclave, pressurized to 0.2 MPa with nitrogen and purged 7 times to remove oxygen, and heated to setpoint under 0.2 MPa nitrogen. After the autoclave charge reached the temperature setpoint (± 4°C), the chamber was pressurized with technical grade hydrogen gas and the organic was stripped for 2 hr with around 500 rpm mixing. The organic was water cooled via cooling coils until the organic was 30 °C or lower, removed, filtered, and the copper product washed 3 times each with acetone and high purity acetone. Copper loosely bound to the reactor was rinsed off with acetone and identified as plastered material, then filtered and washed in the same manner. An ammonia solution (9% NH3) with 20-50 mL of 3% H2O2 heated to 50 °C was used to dissolve the residual copper plating from the autoclave. This was the convention adopted to separately characterize the three copper product forms generated by the procedure: copper powder, plastered copper, and copper plating.
Actual product weight was the sum of the powder and plastered copper weights (total weight recovered), minus the seed weight; compared to the difference between the loaded and stripped organic copper content, which was the strip removal. The copper weight contained in the cleanout solution plus the actual product weight comprised the total weight stripped.
Hydrolytic iron stripping: AB5 nickel-metal hydride batteries, sectioned lengthwise and leached at 10% solids in 6M nitric acid, produced the SX feed solution reported in Table 2. Hydrolytic iron stripping was conducted in the autoclave at 130-160 °C with 25 mL of d.i. water, 500 -1000 mL of loaded organic stirred at 500 rpm, for 60 min after reaching temperature. The steam pressure over the organic at this temperature was 0.17-0.31 MPa. The contents were filtered, and the solids were washed with acetone three times in the filter cone. The filtrate mixture was placed in a separatory funnel to remove the water from the organic after filtration.
Zirconium precipitation stripping: SX organic loading took place by mixing 500 mL of organic with the leachate at an a:o of 1:2. Technical grade oxalic acid ((COOH)2 2H2O) dissolved in d.i. water to a concentration of 1M was heated with the organic at 100-110 °C with 200-250 rpm mixing for 2 hr at an a:o of 1:10 to accomplish the precipitation strip. After separating aqueous and organic phases, the solids were filtered, combined, and washed with acetone and then evaporated to dryness at 100 °C with the residual aqueous solution. Residual metal was removed from the organics to evaluate capacity for reloading and degradation by stripping with 3M NaOH at an a:o of 1:1 for 10 min. After phase separation, the solids were filtered and then rinsed with acetone.
Particle size analysis
Copper particle size distributions were determined by sedimentation rates by a Quantachrome Microscan. The copper product was dispersed in acetone with ultrasonic vibration, then screened at 200 mesh to remove oversized material and filtered. The copper powder was slurried with ethanol and fed to the Microscan which measured the particle settling rates and calculated the sample size distribution and surface area. Stripping product microstructures were examined and photographed using an Hitachi S-530 Scanning Electron Microscope (SEM) with back scattered and secondary electron detectors. Metal components of particles examined in the SEM were identified using an energy dispersive X-ray detector and analyzer.
Discussion of Results
Copper ore Leachate
Precipitation of Cu by hydrogen pressure stripping is reported to be a heterogeneous, autocatalytic reaction, chemically controlled with an activation energy (Ea) of about 113 kJ/mole (Ea >21 kJ/mole; <21 kJ/mole = diffusion controlled) (Chalk, 1959) (Li, 1987). Heterogeneous implies the reaction requires seed addition to prevent plating to reactor surfaces, and autocatalytic denotes self-catalysis by the fresh product forming additional precipitation sites. A summary of temperature, pressure, and modifier effects observed on the characteristics of copper hydrogen pressure-stripped from Kelex extractants was reported by Peterson (1994) in a previous work. In brief, Peterson found plating and plastering losses to be minimized by selecting a strip temperature near 300 °C, and selection of modifier and diluent. High temperature data could not categorically prove that the reaction was homogeneous at higher temperatures, but because of the dramatic drop in plating at this temperature without loss in efficiency, it seems to be possible. Indeed, the reaction proceeds without seed addition at this temperature without any observed increase in organic degradation or loss in strip efficiency.
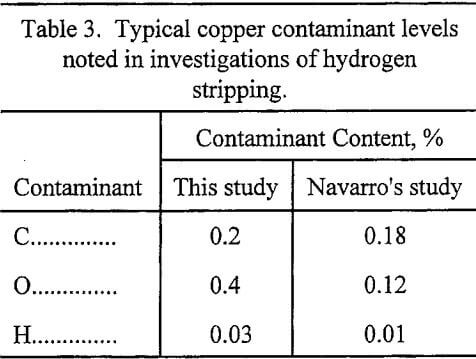
The only contaminants identified in the copper products were carbon, hydrogen, oxygen, and sulfur. The co-loaded iron gradually accumulated in the organic phase, and the leachate gangue metals (“Cycled Organic Leachate” solution from Table 2) did not carry over into the copper products. The contaminants were uniformly distributed throughout the material and were not strictly residual organic on surfaces. This is supported by the fact that impurity levels were not reduced by increased acetone washing or using high purity acetone (no residue after drying). Carbon and oxygen levels, shown in Table 3, were generally higher than reported in previous investigations, possibly due to use of technical grade gas instead of high purity hydrogen and nitrogen (Demopoulos, 1985) (Navarro, 1987).
Cycled organic degradation: Nonylphenol content decreased from 11.4% to 10.5 % (8% by weight of the initial content), while Kelex content in this sample decreased by about 2.2% (22% of initial content) in 14 cycles, with most of the loss occurring in the first few cycles. Decanol content decreased 7.2% (68% of initial
content) after 15 cycles, with the Kelex content dropping about 2.5% (25% of initial content). Nonylphenol concentration remained constant and is the more stable modifier, despite its aromatic character. The organic analyses, performed by the courtesy of Dave GefVert and Witco Industries, appear in Table 4.
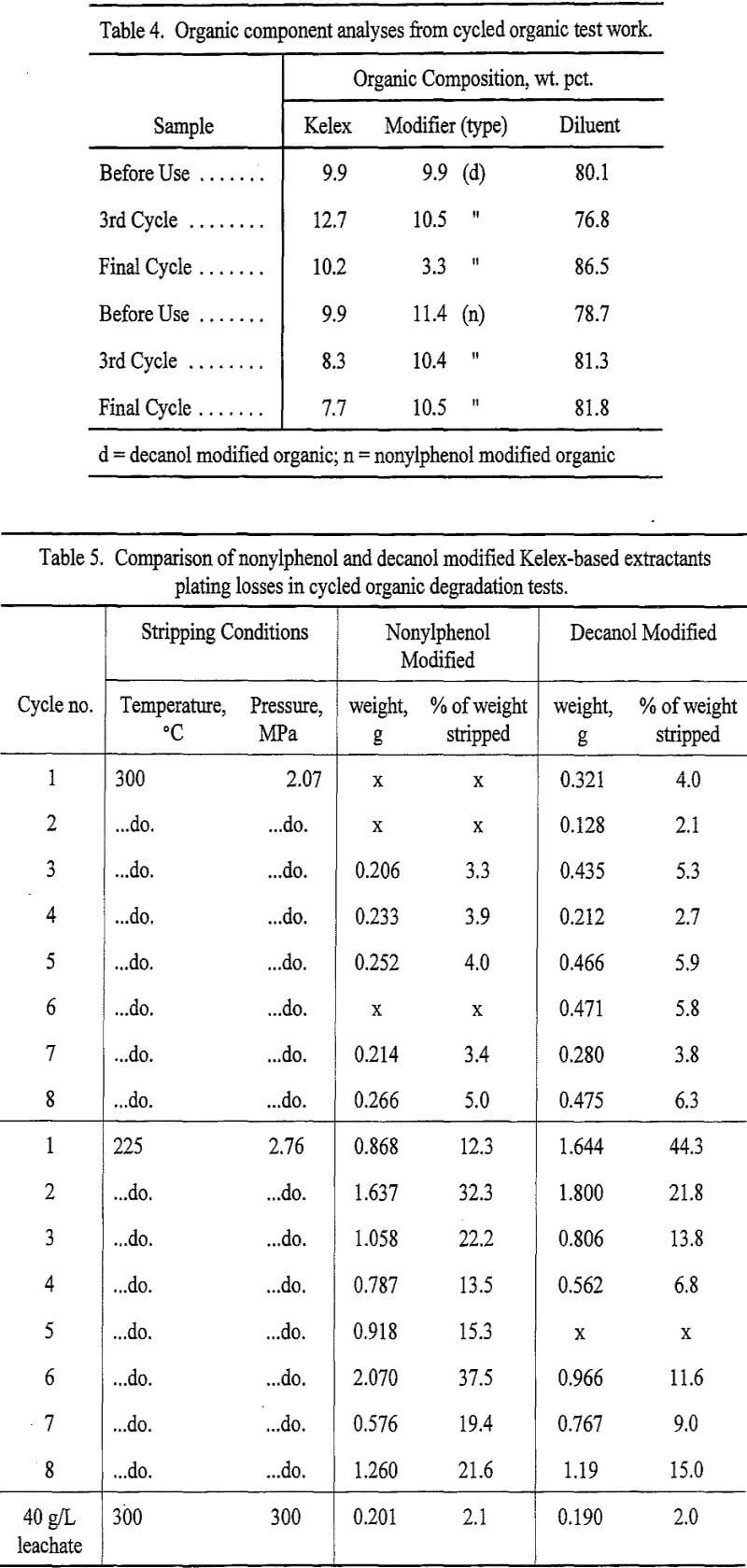
Recycled organic tests examined a decanol modified organic/40 g/L Cu solution derived from the CuSO4 reagent system, and a nonylphenol modified organic/22 g/L Cu ore leachate system at two sets of conditions: 1) 225 °C, 2.76 MPa; and 2) 300 °C, 2.07 MPa. Eight cycles were run at each set of conditions for each organic. The nonylphenol organic was stripped at 300 °C for its first eight cycles and the decanol organic was stripped at 225 °C during its first eight cycles, and then the stripping conditions were swapped for the second set of eight cycles for each organic.
Both organic systems sustained their metal loading capacities throughout the load/strip cycle series. The nonylphenol modified system exhibited the advantages of better phase separation, and lower loss of organic components (indicating higher stability), slightly lower plating losses at 300°C (3.9 wt % vs 4.5 wt %, respectively), and more consistent performance than the decanol modified system. The decanol modified system exhibited lower plating at 225 °C than the nonylphenol modified system at the lower temperature (17 wt % vs 22 wt %, respectively), and lower emulsion formation for a given temperature, but occasional spikes in emulsion formation resulted in higher average amounts of emulsion for both temperature series. The higher temperature produced lowest plating and highest emulsion formation for either organic system. Submicron-sized copper not retained in the filters would coat the phase separation paper in the ensuing test and may have contributed to emulsion formation at the higher temperature tests. Plating losses for the load/strip cycle series and the comparison to the 40 g/L leachate tests are compiled in Table 5 arranged according to strip temperature, while Figure 2 portrays the approximate emulsion volumes and percent plating losses for these tests.
Though attributable in part to modifier and diluent selection, the primary source of the differences in plated material formation for the load/strip cycles is the loaded organic copper content. Higher copper SX aqueous feed content increases the loaded organic copper content, and affects the kinetics of metal removal in the strip. The higher the metal concentration in the organic, the faster the removal rate, the smaller the precipitated particles become, and the more efficient the metal removal becomes.
Examination of plating loss effects using fresh samples of the two organics loaded from the 39 g/L Cu leachate solution produced plating losses of 2 wt % at 300°C for both organic systems. This lower plating loss in the decanol system (from 4.5 to 2.0 wt %) in comparison to the load/strip cycle series indicates an advantage of lower plating with the copper ore leachate replacing the reagent based feed solution. The nonylphenol modified system also improved its plating losses due to the higher copper content of the solution (from 3.9 to 2.1 wt %).
The presence of coloaded iron in the organics may provide some effect which inhibits losses of plated copper by slowing the strip reaction to reduce nonselective attachment to any surface precipitation seed site; or as a catalyst to hydrogen attachment and reduction. Insufficient information on the actual mechanism of hydrogen reduction in the organic phase is available to interpret this effect. In either case, no detriment to product recovery resulted from use of an actual ore leachate based feed solution.
If modifier replacement occurs concurrently with use, decanol should provide better overall performance, but no extended cycle tests were performed to verify this postulate. Either system appears susceptible to the same problem, so overall, the decanol modified system is preferable due to lower product contamination (Peterson, 1994). Nonylphenol consumption would be lower, and both modifiers are similarly priced, the only difference would arise from transportation costs. Decanol currently lists at $1.35/kg, delivered, with nonylphenol ranging from $1.24 – $1.37/kg, f.o.b. east of the Rockies. The actual Kelex losses must be confirmed for decanol to determine the most cost efficient system.
Seed addition: Increasing seed addition levels has been reported to reduce copper plating losses in hydrogen stripping (Demopoulos, 1985). To investigate this further while considering the reduction of plating at elevated temperatures, one liter samples of decanol- and nonylphenol- modified extractants were loaded from a 40 g/L Cu ore leachate derived solution, and stripped at 250 °C and 1.38 MPa hydrogen pressure with no seed copper addition, and with 10 g of copper seed.
These conditions were selected as intermediate from a factorial designed test series which examined temperature (225-275°C) and pressure (0.69-3.45 MPa H2) effects on plating and plastering in the operating region of pressure stripping producing the highest metal losses to these formations. This previous study also identified Exxsol D- 80 as minimizing plastering due to temperature interaction with the diluent, while decanol produced increased plating due to a pressure/modifier interaction (Peterson, 1994).
Metal plating was not practically diminished by increasing seed addition, and plastered material (macroscopic agglomeration of solids with an organic binder) on the reactor components greatly increased with seed addition level (see Table 6). Weight percents plastered and plated (with seed) are based on the total copper weight stripped, which includes copper powder, plastered copper, and plated copper weights. The “percent (no seed)” is based on the total copper weight minus the seed weight added to the test to present a more accurate portrayal of the copper recovery efficiency by the process. Using this basis (no seed) the tests having no seed added exhibit the lowest plating losses and plastering for a given modifier. Nonylphenol modifier produced the lowest plating losses, with or without seed addition, using Kelex-100 in Exxsol D-80.
Plastered material requires rinsing with a solvent to be removed from the autoclave and contains elevated impurity levels, increasing materials handling costs. Metal plated to the reactor requires chemical dissolution for removal and is construed as a material loss in this work, though it could be recirculated back to the solvent extraction step. Seed weight was included with product weight for calculation of weight percent plastered.
Hydrolytic iron stripping
Hydrolytic stripping of iron loaded onto DEHPA from the AB5 battery leach solutions produced low grade iron precipitates containing substantial levels of carbon and phosphorus. The process degraded the DEHPA by consuming phosphorus from the extractant to form an oxy- or hydroxy- organophosphorus compound product between 135 to 160 °C. Results from this testwork are presented in Table 7.
Several unexpected observations were noted in this testwork which are worth mentioning for future reference in the general mechanisms of solvent extractant stripping. No reaction was observed in hydrolytic stripping with DEHPA below 135 to 140 °C. In addition, no Fe precipitation took place in the decanol modified organic unless it was heated to temperature under hydrogen gas. Even seed addition did not initiate stripping at 150 °C unless this hydrogen prereduction took place. This is likely due to reduction of loaded iron from the +3 to the +2 state, which makes the iron easier to strip (Demopoulos, 1984).
The first experiment to produce a precipitate from the unmodified DEHPA organic, AB-4, exhibited radial and acicular crystal formations when observed in the SEM, but subsequent tests did not reproduce any more of the crystalline material illustrated in Figure 3. None of this crystalline material was noted in the decanol modified DEHPA precipitates. Observations in previous investigation of hydrolytic stripping with Versatic acid which did produce some magnetite from stripping fresh Versatic solutions, but recycled solutions only precipitated nonmagnetic hematite (Thorsen, 1979).

Both unmodified and decanol modified DEHPA were degraded as indicated by the high P and C content in the product solids. About 12% of the DEHPA’s phosphorus was consumed from the unmodified organic in 4 cycles while only approximately 10% of the decanol modified organic’s phosphorus was consumed in 6 cycles. Decanol modified DEHPA produced precipitates averaging slightly higher Fe content and lower P content than unmodified DEPHA. Further evidence of this degradation is the variation of product Fe content and variation of the X-ray diffraction (XRD) patterns obtained with the products. Though the patterns recorded definite crystalline structures, they varied between tests and had no definite matches with known patterns. The closest matches were identified as the iron phosphate hydrates lipscombite and strengite with
additional organic material resembling the trace for estrone.
DEHPA is actually composed of two active components: di, 2-ethylhexyl phosphoric acid and mono, 2-ethylhexyl phosphoric acid (MEHPA). The monoalkyl component of DEHPA, which may constitute as much as 50% of the extractant, is more readily stripped of loaded iron, and is more soluble in polar solvents such as water, than the actual di, 2-ethylhexyl component (Demopoulos, 1989) (Acharya, 1988). Due to this higher solubility reported, MEHPA is suspected to be the component in the extractant responsible for producing the iron phosphate precipitates in this study.
Tests AB- 9, 10, 11, and 12 in the decanol modified extractant system recontacted the stripped organic with the combined raffinates from tests AB-6 and 7 to examine coextraction of other metals for potential separation and recovery. Iron extraction appears to become insignificant when the aqueous iron content had been reduced to about 10 g/L, and residuum loaded iron in the organic formed the precipitates for the last 2 cycles. No significant Ni, Ce, or La loading was measured in the organic successively loaded from the previous raffinates. Carbon content of the products is very high despite acetone washing of the filtered solids.
Zirconium precipitation stripping
Recovery and separation of complex metals from an acid waste solution have indicated some potential to separate Nb and Ta from Hf by selection of the solvent extractant modifier. Nonylphenol modified organic loaded Hf but does not extract Nb and Ta; whereas decanol-modified DEHPA did remove Ta and Nb along with the Zr, but rejected most of the Hf. Residual Zr not stripped from the organic by oxalic acid precipitation stripping made up most of the residue obtained by the NaOH strip. Zirconium grades were higher with the NaOH as well, but the residue obtained with the oxalic precipitation contains fewer metallic impurities to complicate subsequent treatments, and the carbon and hydrogen in the oxalate matrix are readily removed by thermal conversion to a high grade oxide. A summary of product grades and solvent extraction data is contained in Table 8.
In general, the precipitation stripped organics were easier to handle because emulsion formation was less than that experienced with the NaOH stripping, but the amount of metal recovered by the precipitation strip was less than that from the NaOH strip. Selectivity for Zr was much higher using the oxalic precipitation stripping with Ti, Ta, and Nb levels below the detection limits in the nonylphenol modified DEHPA mixture. No precipitate was produced with oxalic precipitation stripping from the decanol modified DEPHA. The NaOH strips coprecipitated a significant amount of Ti and Hf, in addition, the decanol modified DEPHA precipitated Ta and Nb with the NaOH strip.
Attempts to precipitation strip Zr from DEHPA using aqueous sulphate solutions were unsuccessful. This method was successful for Zr recovery from the carboxylic acid extractant Versatic acid using reagent grade metal solutions. The Zr-DEHPA ligands are not susceptible to the hydrolysis reaction which allows Versatic extractants to yield Zr oxy-hydroxide precipitates with Mg, Ni, or K sulphate solutions as recorded by Doyle (1992). This method requires the coordination of the bonding in the organic to closely match up with the coordination of the Zr bonding in the precipitated product. The carboxylate bonds are compatible with the partial and complete hydrolysis replacement caused by precipitation stripping whereas the Zr bonding with DEHPA does not maintain an eightfold coordination due to the twin organic chains on DEHPA.
Conclusions
- Decanol or nonylphenol modifier will not prevent DEHPA-based extractants from degradation in hydrolytic stripping as they protect Kelex in hydrogen stripping.
- Oxalic acid precipitation stripping a DEHPA-based extractant loaded from a complex solution containing Zr, Hf, Ti, Ta, and other refractory metals selectively removed Zr without degrading the extractant.
- Seed addition did not improve metal plating losses in hydrogen stripping of Cu from Kelex-based extractants. The seed material tended to plaster onto the reactor components and require rinsing for recovery.
- Nonylphenol-modified Kelex organic extractants produced higher initial metal loadings than decanol-modified extractants, but resulted in extractant losses in repeated load/strip cycles.