Table of Contents
A sulfuric acid leach technique for extracting gallium and germanium from Apex ore without the use of a reducing agent, such as SO2, was investigated. Leaching studies showed that with increasing sulfuric acid concentrations, gallium extraction increased to over 95%, while germanium extraction reached a peak of 71% before decreasing to less than 50%. It was hypothesized that at high acid concentrations, germanium precipitated from solution as GeO2.
For optimum gallium and germanium extraction, a two-stage counter-current leach system was devised to adequately control the solution acidity. At operating conditions of 1,000 kg H2SO4/mt ore, 20 % solids, and 90 °C, 96% of the gallium and 85% of the germanium in the ore were extracted. Microprobe studies showed that approximately 10 to 15% of the germanium in Apex ore is in solid solution with silica and cannot be leached with sulfuric acid or SO2.
———–
The Apex Mine near St. George, Utah, USA, is the only ore body in the world which has been mined primarily for germanium and gallium within the last ten years. From 1880 to 1945, copper was extracted from this unique property, leaving behind unmined iron-rich minerals containing germanium and gallium. Geological studies of the site (Bernstein, 1986, Dutrizac, 1986) have revealed that germanium is concentrated mainly in goethite, while gallium is found chiefly in jarosite. It is postulated that at some geological time, germanium and gallium substituted for iron in these minerals. Therefore, to extract germanium and gallium from the ore, it is necessary to solubilize the iron compounds.
Musto Explorations LTD, former owner of the mine, employed a 3-stage counter-current process using sulfuric acid and SO2 to leach approximately 80% of the germanium and 90% of the gallium from the ore (Olsen, 1989). Researchers (Swinkels, 1986) commissioned by Musto determined that a reducing agent, such as SO2 , must be present along with sulfuric acid to attack goethite according to reaction A. Jarosite could be attacked by SO2 alone according to reaction B.
2FeOOH + SO2 + H2SO4 = 2FeSO4 + 2H2O…………………………………………………….(A)
2KFe3(SO4)2 (OH)6 + 3SO2 = 6FeSO4 + K2SO4 + 6H2O……………………………………(B)
Jiang, et al (1988-89) in their sulfuric acid leaching studies of this ore also maintained that a reducing agent was needed for high germanium and gallium extractions. These researchers were able to leach approximately 95% of the gallium, but only up to 65% of the germanium.
Previous work (Harbuck, 1989) by the Bureau of Mines on sulfuric acid leaching of germanium and gallium from zinc residues showed that although the addition of SO2 increased extraction, the same effect could be achieved with increased sulfuric acid concentration. Because germanium and gallium in both zinc residues and Apex ore are tied up in iron compounds, it was felt the previous research could be applicable to this ore.
According to reactions C and D, goethite and jarosite can be attacked with sulfuric acid alone.
2FeOOH + 3H2SO4 = Fe2(SO4)3 + 4H2O…………………………………………….(C)
2KFe3(SO4)2 (OH)6 + 6H2SO4 = 3Fe2(SO4)3 + 12H2O + K2SO4…………..(D)
Comparing reaction A with C and B with D, it is seen that the addition of SO2 to the sulfuric acid reduces the total moles of leaching reagents needed to attack the iron compounds. However, because SO2 is approximately six times more expensive than sulfuric acid, the cost of reagents would be considerably less and the overall leaching process would be simplified if only sulfuric acid were used. In addition, SO2 is difficult to handle and environmentally hazardous. Therefore, it was the goal of this research to study the extraction of germanium and gallium from Apex ore using only sulfuric acid and eliminate the use of SO2.
Material and Equipment
Ore samples were obtained from Hecla Mining Company, current owners of the Apex Mine. Analyses of the ore using inductively coupled plasma spectrophotometry (ICP), neutron activation analysis (NAA) and atomic absorption spectroscopy (AA) are given in Table 1.
Batch agitation leach tests at various experimental conditions were performed using standard laboratory hotplates, thermocouples, stirring devices, flasks, and glassware.
Results and Discussion
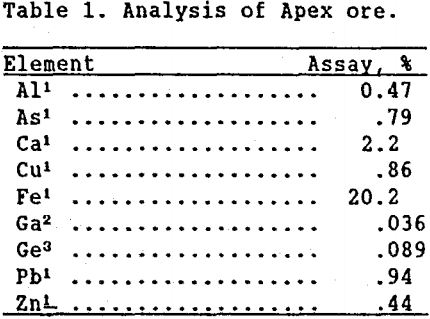
Sulfuric acid leaching of Apex ore was initially tested at 20% solids for 2 hr at 80°C. Figure 1 shows the effect of increasing the initial sulfuric acid concentration on germanium, gallium, and iron extraction. Since geological studies maintained that germanium and gallium are uniformly distributed in the iron lattices (Bernstein 1986), and since goethite and jarosite are both ferric iron minerals, it was expected that the three extraction curves would follow each other fairly closely. As seen, gallium and iron extractions increased to over 95% as the sulfuric acid concentration increased, indicating the relatively high solubility of these metals. Germanium extraction, however, reached a peak of 71% using 5M H2SO4. Above this point, germanium extraction decreased with increasing sulfuric acid concentration, indicating the precipitation of solubilized germanium from the leach solution. This initial testing showed that while high gallium extraction from Apex ore was easily attainable by increasing the sulfuric acid concentration in the leach liquor, germanium extraction warranted further investigation.
Germanium Extraction
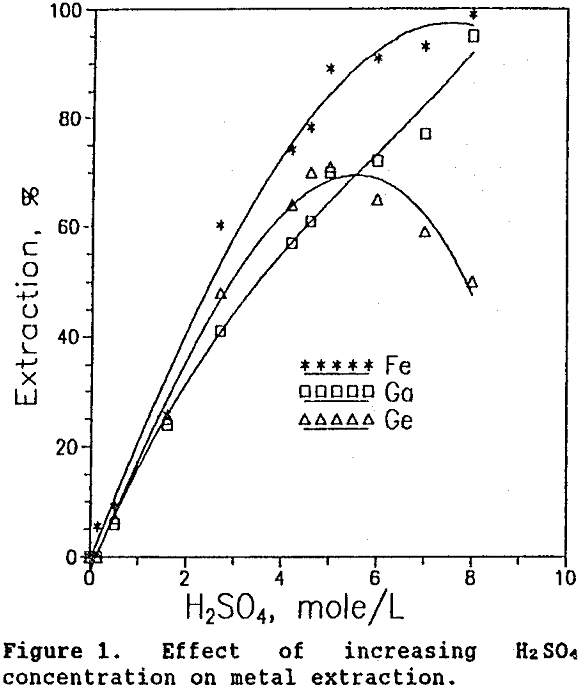
From Figure 1 it is apparent that in the leaching of Apex ore, germanium solubility decreased substantially in highly concentrated sulfuric acid solutions. This same behavior was observed in the leaching of germanium from zinc processing residues (Harbuck, 1989). Pugh (1929), in studying the GeO2-H2SO4 system at 25°C, Figure 2, found that as the sulfuric acid concentration increased, germanium concentration in solution decreased. (It should be noted that the dissolution of GeO2 is a slow process. Each data point in Figure 2 required 8 to 10 days agitation before equilibrium was attained.)
In his studies, Pugh concluded that germanium sulfate is incapable of existence, a conclusion which has been substantiated through the years (JCPDS, 1989). Therefore, germanium must be precipitating from concentrated sulfuric acid solutions as GeO2. A simple experiment was performed verifying this assumption. Five grams of 99.99% pure, commercially purchased GeO2 were dissolved over time in 2 L of water at ambient temperature. As increasing amounts of sulfuric acid were added to this solution, a white precipitate formed. X-ray diffraction (XRD) analysis identified the compound as GeO2. Likening these results to Figure 1, it could be concluded that in the leaching of Apex ore, the addition of increasing amounts of sulfuric acid forced the precipitation of solubilized germanium as GeO2.
However, there is the possibility that in actual leach solutions many impurities exist which could combine with germanium to form precipitates. To test this possibility, sulfuric acid was added to an actual Apex leach filtrate. Because the filtrate contained only 0.27 g/L Ge, a germanium spike solution (made by dissolving 99.99% pure GeO2 in water) was also added, increasing the germanium concentration to 2.8 g/L. When increasing amounts of sulfuric acid were added to this mixture, a white precipitate formed. XRD analysis verified that the precipitate was GeO2.
Therefore, it is postulated that when Apex ore is leached, sulfuric acid attacks the goethite and frees the germanium. However, during the leach (and before complete dissolution of germanium from the ore), as the sulfuric acid concentration becomes too high, part of the solubilized germanium precipitates as GeO2.
Avoiding Germanium Precipitation
To avoid germanium precipitation during the leaching of Apex ore, the amount of sulfuric acid in the leach solution must be controlled. If too much acid is added, germanium will begin precipitating from solution before the dissolution of germanium from the ore is complete. If not enough acid is added, germanium will not be freed from the goethite lattice. In either case, the end result is low germanium extraction.
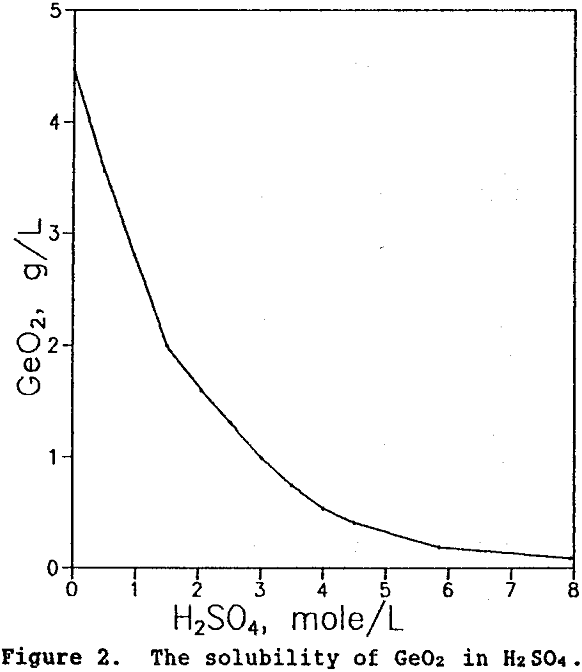
To obtain high germanium extraction at the lowest possible sulfuric acid concentration, other variables such as leach temperature and time were examined. The effect of increasing leach temperature from 25 to 100°C is shown in Figure 3 for tests performed for 2 hr with a 2.2M H2SO4 solution. As seen, the highest germanium extractions were obtained at temperatures between 90 and 95″C.
Time tests were conducted at 90°C and 20% solids at three different acid concentrations: 2.6M, 5.3M, and 8.0M. The corresponding germanium and iron extraction curves are shown in figures 4, 5, and 6. The highest germanium extraction of 85% was attained after 72 hr using the 2.6M H2SO4 leach solution. For comparison, germanium extractions with the 5.3M and 8.0M H2SO4 leach solutions decreased to 78% and 37%, respectively after 72 hr. More importantly, because germanium extraction continued to follow iron extraction in the 2.6M H2SO4 test, it is clear that germanium was not precipitating from solution, as it clearly was in the 5.3M and 8.0M H2SO4 test.
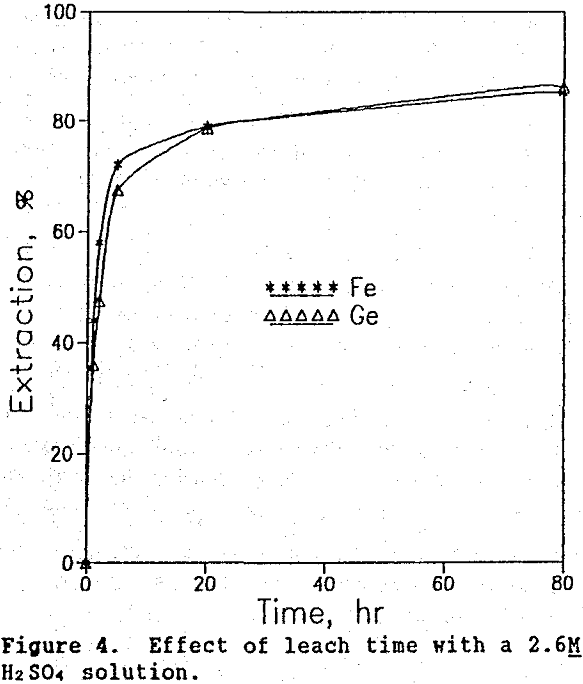
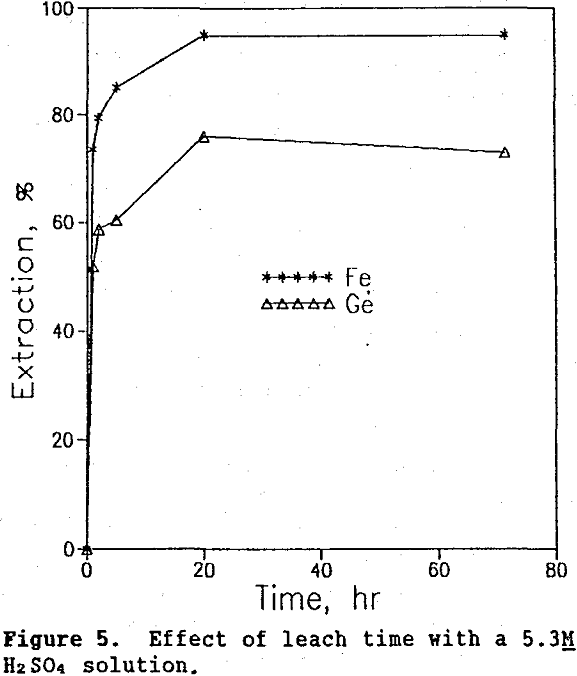
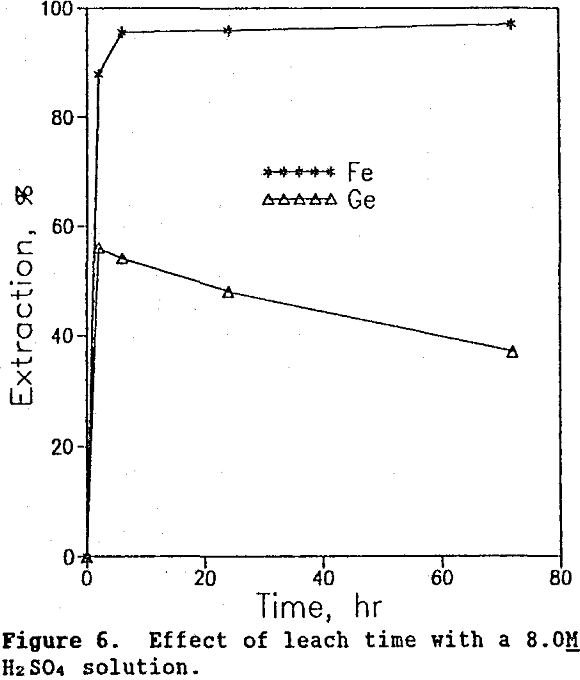
Thus, the use of lower sulfuric acid concentrations over longer leach times increased germanium extraction to 85%, a level 5% higher than that obtained in the system using both sulfuric acid and SO2 (Olsen, 1989).
Two-Stage Counter-Current Leaching
Based on these results, a two-stage counter-current leach system as shown in Figure 7 was proposed for extracting high amounts of both germanium and gallium from Apex ore.
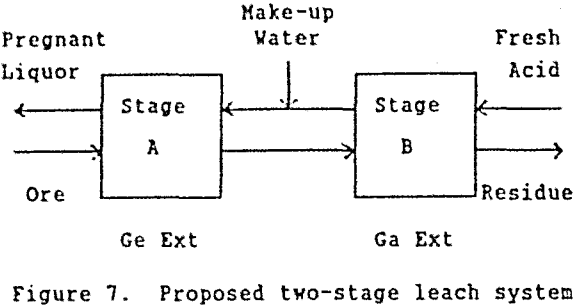
In stage A, used acid from stage B contacts fresh ore for a relatively long leach tine (at least 20 hr) to extract high amounts of germanium. In stage B, fresh acid contacts previously leached ore for a shorter period of time (approximately one-third the time of stage A) to extract relatively high amounts of gallium. The acid concentration in stage B must be sufficiently high to extract most of the gallium. However, because of the counter-current action, the acid concentration can be considerably less than shown in Figure 1 (i.e. 8M H2SO4). Since most of the germanium would be solubilized in stage A, a high acid concentration in stage B would not adversely affect germanium extraction.
This system was tested using a leach time of 20 hr in stage A and 6 hr in stage B with a temperature of 90°C and 20 % solids in each stage. The pulp density in stage B was based on the actual weight of dry solids remaining after the stage A leach. Varying amounts of sulfuric acid were added to determine the optimal amount of acid needed for both high gallium and germanium extractions. The results shown in Table 2 indicate that germanium extraction remained fairly constant as the initial acid concentration in stage A decreased from 3.2 to 1.9M. However, when the initial acid concentration in stage B decreased from 4.9 to 2.9M, gallium extraction dropped from 97 to 70%. Table 2 also lists the total amount of acid used on a kg/mt basis. At 20 % solids, at least 1,000 kg H2SO4/mt ore were required for both high gallium and germanium extraction.
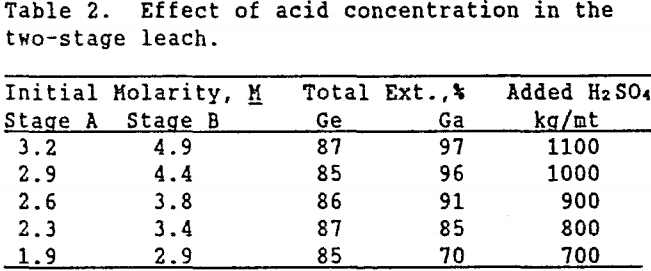
Increasing the pulp density of the leach slurry was studied as a method to use acid more efficiently (less sulfuric acid to maintain the same acid concentration). Table 3 shows the effect on metal extraction when the initial % solids in Stages A and B were 25% and 30%, respectively.
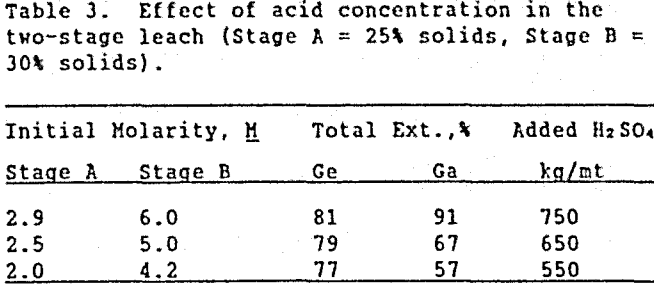
Increasing the pulp density of the two-stage leach did not significantly reduce the amount of acid needed (per metric ton of ore) to leach high amounts of gallium and germanium. However, increasing the pulp density did significantly increase the iron concentration in solution from an average of 55 g/L (with 20 % solids) to 90 g/L Fe (with 30 % solids), approaching iron saturation. To avoid this problem, the two-stage, 20 % solids leach is recommended. It should be realized that these tests show trends on a laboratory scale. To use the information on an industrial level, pilot plant studies must be performed.
Increasing Germanium Extraction
The highest germanium extraction attained, even under optimum two-stage leaching conditions, was 87% (see Table 2). A key to increasing germanium extraction is understanding the mineralogy of the ore. In this study, microprobe studies were performed on the ore before and after leaching. Individual grains of the original and leached ore (residue) were scanned with the electron beam and analyzed for germanium, iron, and silicon. In the ore, a major portion of the germanium was found to be associated with iron while approximately 10 to 15% of the germanium was associated with quartz. In the residue, germanium was no longer associated with iron, but was found in solid solution in individual quartz (silica) grains. This information suggests that 10 to 15% of the germanium in Apex ore is tied up with silica, and cannot be leached with sulfuric acid.
Gruzdev and Vydrin (1967) have documented the affinity germanium has for silica. Miller, et al. (1963) have demonstrated how GeO2 can substitute into the four-fold coordinated structures eof silica. It is theorized that in the formation of this deposit, some germanium substituted into silica grains. This theory was further verified by leaching the residue in a HF solution. Since HF attacks silica, leaching with HF should destroy the silica solid solution, and solubilize the germanium. Table 4 shows that as the HF concentration in the leach solution increased, germanium solubility increased, resulting in a total of 99% of the germanium in Apex ore being extracted.
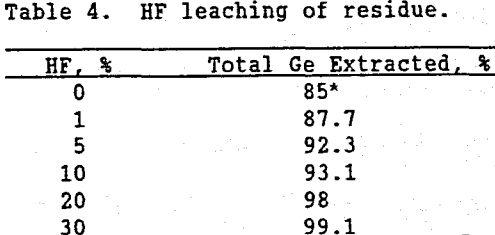
Because of the higher reagent cost and related environmental and safety problems associated with HF, recovering the remaining 10 to 15% germanium with HF would probably not be an economic option.
