Table of Contents
Standard tellurium analytical procedures were modified to obtain the greater sensitivity needed to support a survey of tellurium resources by the Bureau of Mines. Resulting volumetric and spectrophotometric methods for determining tellurium in all types of tellurium-containing materials are described. Sample decomposition and preliminary separations of interfering selenium, gold, and silver are the same in both procedures.
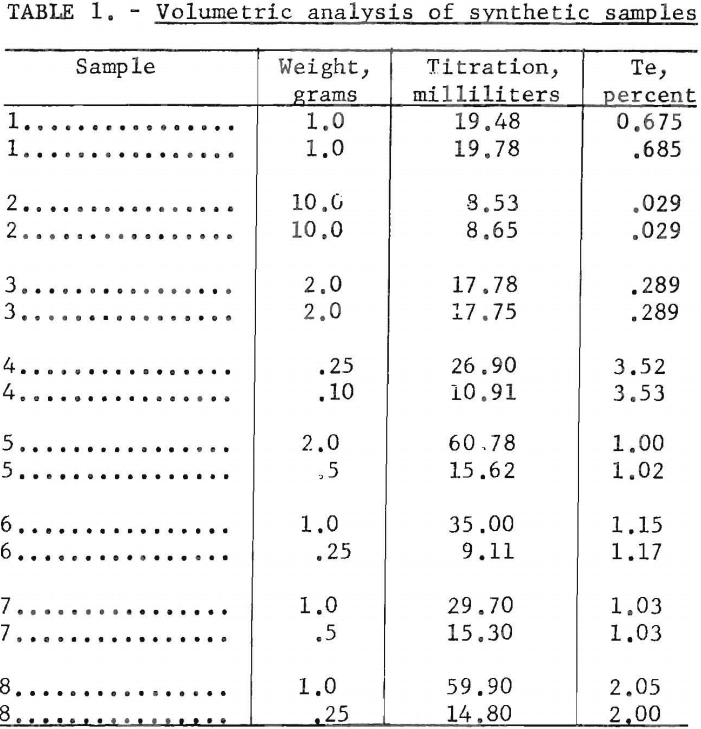
The volumetric method is applicable to samples containing a minimum of 10 parts per million of tellurium. In this method the tellurium is reduced to elemental form, and the colloidal suspension is titrated with 0.01 normal iodine solution. The weight of sample for analysis, from 0.25 to 10 grams, should be selected so that the sample contains 0.1 to 5 milligrams of tellurium. To insure accuracy, duplicate samples should be run and the results averaged, particularly on samples of low tellurium content. The limit of determination is 0.001 percent tellurium, which is equivalent to 0.1 milligram of tellurium when using a 10-gram sample.
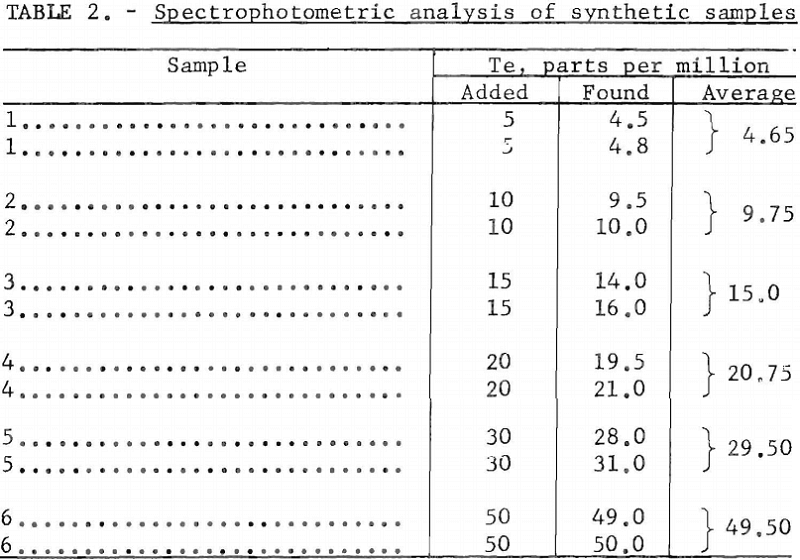
The spectrophotometric method is particularly applicable to low-tenor materials containing from 0,5 to 50 parts per million of tellurium. Up to 10 gram samples are required for making accurate determinations in the low concentration range. The sample used should contain between 5 and 100 micrograms of tellurium. The turbidity of a colloidal suspension of tellurium prepared from such a sample is determined in a spectrophotometer at a wavelength of 440 millimicrons, and this value is compared with those of standard samples containing known amounts of tellurium.
Owing to new interest in tellurium for use in thermoelectric devices, the Bureau of Mines has been engaged in a survey of tellurium resources. Samples submitted for tellurium analyses ranged from anode slimes and smelter fumes containing 1 percent or more tellurium to ores, slags, and rocks containing a few parts per million or less of tellurium. Routine tellurium analytical procedures lacked the precision and sensitivity needed, particularly in the part-per-million range. Hence, modifications were devised to obtain the desired improvements.
Routine methods for determining tellurium are (1) gravimetric, weighing as the metal or the oxide; (2) volumetric, titrating with iodine or potassium dichromate ; and (3) spectrophotometric. Large amounts of tellurium usually are determined by gravimetric or volumetric methods; trace amounts, by turbidity methods, using a spectrophotometer to measure the absorbance.
To attain the desired precision and sensitivity, the volumetric method of Evans and the spectrophotometric method of Hanson were modified by application of selenium, gold, and silver separation procedures, and by adopting techniques for handling samples as large as 10 grams. Sample decomposition and separation of interfering elements were the same for both the volumetric and spectrophotometric methods. The preferred procedures currently used at this Bureau of Mines Center are detailed in this report.
Accuracy of the Methods
The determination of the accuracy of the methods subsequently to be described proved to be a problem, as analyzed standard samples were unavailable. However, this problem was surmounted by preparing synthetic standard samples. These samples were made by adding known amounts of tellurium to tellurium-free gangue material similar to samples sent in for analysis. The synthetic standard samples then were analyzed as indicated.
The accuracy of the volumetric method used in the higher concentration ranges is good. Typical results obtained in replicate determinations are given in table 1.
The spectrophotometric method, usable in the low concentration range of 0.5 to about 50 parts per million tellurium, also yielded accurate results. Determinations made on synthetic samples, prepared as noted, yielded the data given in table 2.
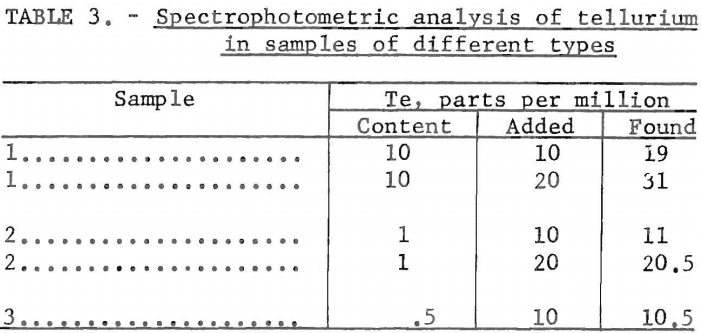
In other samples, also analyzed spectrophotometrically, known amounts of tellurium were added to samples that previously had been analyzed and were known to contain the element. Several samples of different types of composition were used. The total tellurium contents determined were summations of the naturally contained tellurium plus the added tellurium. The results obtained are given in table 3.
Sample Decomposition and Separation of Interfering Elements
Decomposition
Weigh an appropriate-size sample (0.25 to 10 grams) into a 400-milliliter beaker. Add 5 to 20 milliliters of nitric acid and digest on a hotplate. Add 10 to 20 milliliters of sulfuric acid and evaporate to heavy fumes. This treatment will suffice for most samples. If the sample has been subjected previously to high temperatures, part of the tellurium may have been converted to a form not readily decomposed by nitric and sulfuric acids. On such samples complete decomposition can be effected by further addition of 5 to 20 milliliters of hydrofluoric acid and 10 to 20 milliliters of sulfuric acid, and evaporation to heavy fumes. The beaker then should be removed from the hot-plate, cooled, the sides washed down with distilled water, and the solution again evaporated to heavy fumes to remove fluorine.
Separation of Selenium
Cool the sample and transfer to a standard 500-milliliter distillation flask by flushing out the beaker with approximately 20 milliliters of water. Add 40 milliliters of hydrobromic acid; connect the distillation apparatus; and adjust the tip of the condenser so that it wall dip below the surface of a 400-milliliter beaker, containing 20 milliliters of water and 5 milliliters of hydrobromic acid. Distill the selenium at an even boil, adding boiling chips if necessary. Continue the distillation until the sample is just short of fuming. The distillate may be discarded or reserved for selenium determination.
Transfer the residual solution containing the tellurium from the distillation flask to the original 400-milliliter beaker, rinse out the flask with 10 milliliters of hydrobromic acid, an excellent solvent for tellurium, and then with distilled water. Filter off the insoluble residue through a medium filter paper, precoated with paper pulp, into a 400-milliliter beaker, and wash three or four times with hot distilled water. To the filtrate add 50 milliliters of concentrated hydrochloric acid and 2 milligrams of arsenic in the form of arsenious acid solution. The arsenic serves as a carrier for the tellurium should the content be extremely low. Bring the solution to a boil on a hotplate at medium heat, and cautiously add 10 milliliters of 30-percent hypophosphorus acid (H3PO2) solution. All of the tellurium in the solution is reduced to elemental tellurium (Te°) by HaPO2, a strong reducing agent. The added arsenic, as well as any mercury, silver, gold, platinum, and palladium that may be present, also is reduced to elemental form. Boil the sample for about 5 minutes, then filter through a Baker and Adams,” grade “0” or equal, filter paper precoated with paper pulp. Wash three times with hot 1:1 hydrochloric acid and then three times with hot distilled water.
Transfer the filter paper containing the tellurium, arsenic, gold, and other metals to the original beaker; add 20 milliliters of nitric acid and 10 milliliters of sulfuric acid. Cover the beaker with a watchglass, and heat to fumes. Add more nitric acid if needed to complete the decomposition of the filter paper. Wash down the sides of the beaker and the watchglass and again heat to fumes. Cool the sample and dilute it to approximately 80 milliliters Place the beaker on a hotplate and bring the solution to a near boil.
Separation of Gold and Silver from Tellurium
To the hot solution add hydrogen peroxide by drops to precipitate the gold as elemental or metallic gold. Hydrogen peroxide will reduce aurous or auric sulfate to metallic gold, as shown in equations 1 and 2
Au2SO4 + H2O2 → 2Au + H2SO4 + O2……………………………………(1)
Au2(SO4)3 + 3H2O2 → 2Au + 3H2SO4 + 3O2………………………….(2)
Hydrogen peroxide acts as a strong reducing agent toward gold, but it does not affect the tellurium because of the widespread difference between the oxidation-reduction potentials of gold and tellurium. These differences can be seen clearly in the following equations as given by Latimer (9):
2H2O + Te = TeO2 + 4H+ + 4e E° = -0.529 volts…………………..(3)
H2O2 = O2 + 2H+ + 2e E° = -0.68 volts……………………………….(4)
Au = Au+3 + 3e E° = -1.50 volts……………………………………………(5)
Au = Au+ + e E° = -1.68 volts……………………………………………….(6)
Heat the solution to boiling, and boil for several minutes to coagulate the gold. Add a few drops of hydrochloric acid to precipitate any silver as silver chloride and continue boiling for a few more minutes. Filter off the gold and silver by means of a fine-porosity filter paper precoated with paper pulp and wash four times with hot water.
The filtrate from the gold and silver separation step, which should have a volume of about 100 milliliters, contains tellurium and any platinum and palladium that may be present. These elements do not interfere with the subsequent tellurium determination. To the filtrate add 50 milliliters of concentrated hydrochloric acid and approximately 1 milligram of arsenic as arsenious acid. Place the beaker on a hotplate, add a boiling chip, and bring the solution to a boil. Add 10 milliliters of hypophosphorus acid to precipitate the tellurium as elemental tellurium, and continue boiling for 5 minutes. Cool the solution to about 60° C, and filter through a fine-grained filter paper precoated with paper pulp. Wash three times with 1:1 hydrochloric acid and three times with water. If a black precipitate can be seen on the filter paper, enough tellurium is present to be determined volumetrically. If only a small amount of brown precipitate can be seen, only a trace amount of tellurium is present, and the tellurium should be determined spectrophotometrically.
Volumetric Method
The volumetric method is based on the reduction of tellurium to a colloidal suspension of elemental tellurium. The elemental tellurium (Te°) once isolated is oxidized to tetravalent (Te+4) tellurium by titrating with standard 0.01 normal iodine solution in accordance with equation 7.
Te + 2I2 + 3H2O → H2TeO3 + 4HI……………………..(7)
1.0 milliliter of 0.01 normal iodine = 0.000319 gram of tellurium
Place the filter paper containing the tellurium, as described in the preceeding section, in a 125-milliliter Erlenmeyer flask, and add 20 milliliters of nitric acid and 5 milliliters of perchloric acid. Cover with a small watch glass, place on a hotplate, and digest at a medium heat to decompose the filter paper. The initial decomposition of the filter paper may be quite violent, and care should be taken to prevent boiling over. When the initial action has subsided, rapidly evaporate the sample to soft dryness over a burner.
Allow the sample to cool, and then wash down the cover, sides, and lip of the flask with water. Add 1 drop of phenolphthalein, and neutralize the solution with a few drops of a 50-percent sodium hydroxide solution. Add 5 milliliters of phosphoric acid and 5 milliliters of 1-percent acacia (gum arabic), and dilute to 50 milliliters with water. Add 2 to 3 grams of sodium hypophosphite, and heat just to the boiling point. As the boiling point is approached, the solution will darken suddenly, owing to the formation of a fine colloidal suspension of elemental tellurium. The suspension is so fine that the solution appears homogeneous and will be light brown to black, depending upon the amount of tellurium present. If less than 1 milligram of tellurium is present, the solution will be light brown; if there is more than 3 milligrams of tellurium, it will be black. Place the beaker in a cooling tray, and cool to about 15° C. Dilute to 100 milliliters with cold water. The sample now is ready for titration.
Titrate the sample with a standard 0.01 normal iodine solution. Add the iodine solution slowly with vigorous shaking between each addition until the solution is almost colorless. Add 15 milliliters of carbon tetrachloride, and continue the titration a drop or two at a time with vigorous shaking. The carbon tetrachloride layer will become lighter as the endpoint is approached and will be colorless when all of the tellurium is oxidized, A drop or two of iodine in excess will impart a pink tint to the carbon tetrachloride layer. This is the endpoint indication of the titration.
Subtract 0.1 milliliter for a titration blank, and calculate the amount of tellurium present. A reading of 0.3 milliliter is equivalent to 0.1 milligram tellurium or 10 parts per million (0.001 percent) in a 10-gram sample. This amount of tellurium is considered to be the minimum concentration that can be determined reliably by the volumetric method.
Spectrophotometric Method
The spectrophotometric method is based on the reduction of tellurium to colloidal form by stannous chloride. The quantity of tellurium present is determined by measuring the light absorption of the solution in comparison with that of solutions containing known amounts of tellurium.
Procedure
Place the filter paper containing the tellurium, prepared as described in the section on sample decomposition and separation of interfering elements, in a 250-milliliter beaker. Add 20 milliliters of nitric acid and 5 milliliters of perchloric acid, decompose on a hotplate, and fume to soft dryness. Cool and add 3 milliliters of hydrochloric acid. If the solution is clear, transfer to a 10-milliliter volumetric flask and dilute to 9 milliliters; if it is not clear, filter into a 10-milliliter flask and adjust the volume to 9 milliliters. Add 1 milliliter of 10-percent stannous chloride solution and shake. The tellurium is reduced to a fine colloidal precipitate of elemental tellurium. Transfer a portion of the solution to a 1-centimeter absorption cell and read the turbidity on a spectrophotometer at a wavelength of 440 millimicrons and a slit width of 0.02 millimeter. To adjust the machine preparatory to measuring the light transmittance of sample solutions, use a solution of water, hydrochloric acid, and stannous chroride similar to the sample in the reference cell. A reading corresponding to 5 micrograms tellurium is equivalent to 0.5 part per million tellurium (0.00005 percent) if a 10-gram test sample is used. This is the minimum concentration that can be determined reliably by the spectrophotometric procedure.
Standard Curve for Spectrophotometric Determinations
Prepare a tellurium solution by dissolving 1 gram of pure tellurium metal in 15 milliliters of nitric acid, add 10 milliliters of perchloric acid, and take to soft dryness. Add 10 milliliters of hydrochloric acid, and dilute to 1 liter. One milliliter of this solution contains 1 milligram of tellurium. From this standard solution prepare two dilute standard solutions by pipetting 5 and 20 milliliters, respectively, into two 1-liter volumetric flasks and dilute to the mark with water. These solutions contain 5 and 20 micrograms of tellurium per milliliter, respectively.
Prepare a series of standards containing 5 to 100 micrograms of tellurium by pipetting appropriate aliquots of the dilute standard solutions into 10-milliliter volumetric flasks. Add 3 milliliters of hydrochloric acid and 1 milliliter of 10-percent stannous chloride solution; dilute to the mark, and shake. Transfer to 1-centimeter absorption cells, and read the absorbance as in the regular method. Plot a curve showing micrograms of tellurium versus absorbance.
Reagents
Reagent grade chemicals of the highest quality available should be used in the analyses. Chemicals required are listed as follows:
- Acids:

- Salts and chemical solutions:
Arsenious acid solution (H3AsO3). Dissolve 265 milligrams As2O3 in 3 milliliters of 50-percent sodium hydroxide solution, make just acid with H2SO4, add 5 milliliters excess acid, and dilute to 500 milliliters (5 milliliters contains 2 milligrams As).
Sodium hypophosphite (NaH2PO2)
Sodium hydroxide solution (NaOH) 50 percent
Hydrogen peroxide solution (H2O2) 30 percent
Stannous chloride solution (10 percent). Dissolve 100 grams SnCl2 in 150 milliliters hydrochloric acid, and dilute to 1 liter.
Iodine solution (0.01 normal). Dilute 100 milliliters of standardized 0.1 normal iodine solution to 1 liter.
Phenolphthalein. Dissolve 1 gram in 50 milliliters of alcohol and 50 milliliters water.
Gum arabic solution, 1 percent in water.
Carbon tetrachloride (CCl4).
