Table of Contents
Titanium dioxide or titania pigment is prepared commercially from ilmenite, an iron-titanium oxide mineral, by the sulfate process. Ilmenite is digested with H2SO4 to yield a titanium sulfate solution. Subsequently, the solution is hydrolyzed to yield a hydrous titania precipitate that is calcined to produce pigment. A major problem associated with the process is the generation of large quantities of byproduct iron sulfate and H2SO4, which are difficult to dispose of in an environmentally acceptable manner. The promulgation of environmental protection regulations throughout the world has forced operators of titania pigment plants to develop new, costly waste- disposal methods. Sorelslag prepared by arc-smelting of ilmenite also is used commercially in the sulfate process to produce titania pigment. The Sorelslag, containing about 70 wt-pct TiO2 and 10 to 15 wt-pct iron oxide, still yields iron sulfate as a byproduct in the sulfate process; consequently, disposal problems are reduced but not eliminated.
One of the goals of the Federal Bureau of Mines is to minimize the undesirable environmental conflicts, impacts, and occupational hazards associated with the processing of minerals. In support of this goal, the present research was concerned with developing a flux-smelting technique to yield sodium titanate product and a pig iron coproduct from ilmenite. The titanate product would be used as a feed material to the sulfate process for preparation of titania pigment, thus avoiding ferrous sulfate waste-disposal problems.
In earlier work, a borate-smelting technique was investigated. Borate smelting yielded pig iron and a sodium titanate product containing less than 1 wt-pct iron. Because cost evaluation showed that the use of sodium borate as a smelting flux was uneconomical, soda ash was used as the flux in subsequent investigations.
The work covered in this report evaluates the sodium titanate product obtained from soda smelting as a feed material for the sulfate process to produce titania pigment. The evaluation of the use of a low-soda titanate (LST) as feed for the sulfate process and the regeneration of acid solutions for reuse are also included.
Preparation of Titanate Intermediate Products
The sodium titanate intermediate products used in this investigation as feed material for the sulfate process were obtained by soda smelting a New York rock ilmenite concentrate, which contained about 45 wt-pct each of TiO2 and FeO. One part ilmenite, 0.25 part soda ash, and 0.1 part carbon were smelted at 1,300° C to yield pig iron and slag. The molten slag was quenched and water leached, and the pulp was filtered to yield a high-soda titanate product (HST) having the following analysis, in weight-percent: TiO2, 66 to 75; Na2O, 19 to 21; Fe2O3, <1; MgO, 3 to 6; SiO2, 2 to 4; Al2O3, 1 to 3; and CaO, approximately 1. A flowsheet for the preparation of HST from ilmenite is shown in figure 1.
Another titanate product, LST, was prepared from the HST by the following technique : 1 part HST was blended with 0.9 part 36-wt-pct H2SO4, and the mixture was heated at 500° C for 2 hours. The calcine then was quenched and leached in water at 70° to 80° C for 2 hours. The pulp was filtered to yield a LST product having the following analysis, in weight-percent:
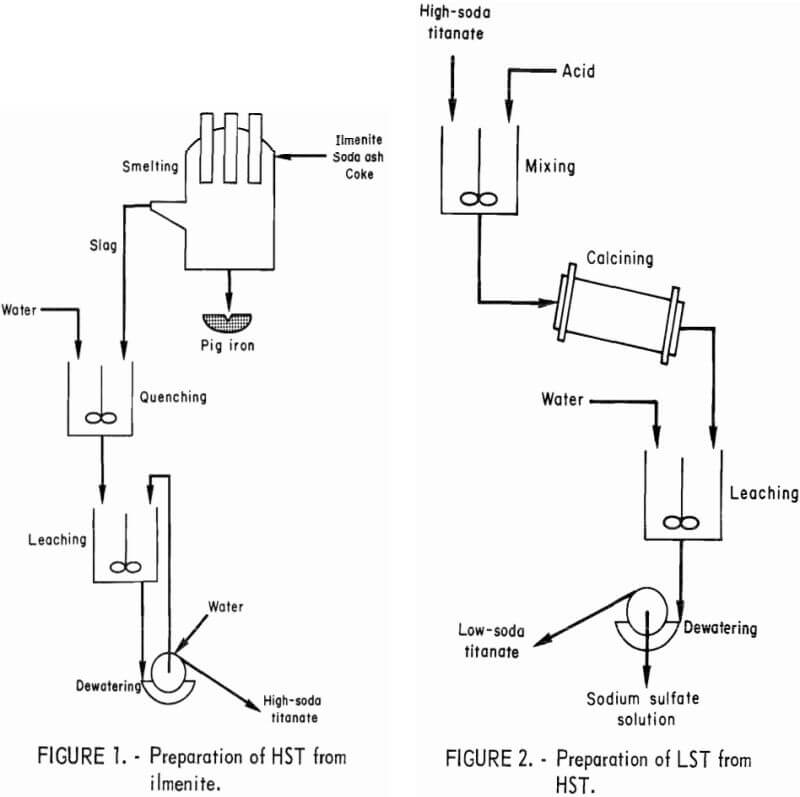
TiO2, 78 to 89; Na2O, 0.1 to 2.0; Fe2O3, 0.3 to 0.7; SiO2, 2.0 to 5.0; and CaO, 1.0. The procedure for preparing LST from HST is shown in figure 2.
Utilizing LST as feed material for the sulfate process would greatly decrease the soda buildup in the process liquors, thus minimizing problems associated with regeneration of the acid solutions. This LST might serve also as a rutile substitute for feed material to the chloride process, which is also a process used industrially for producing titania pigment.
Simulated Sulfate-Process Experiments
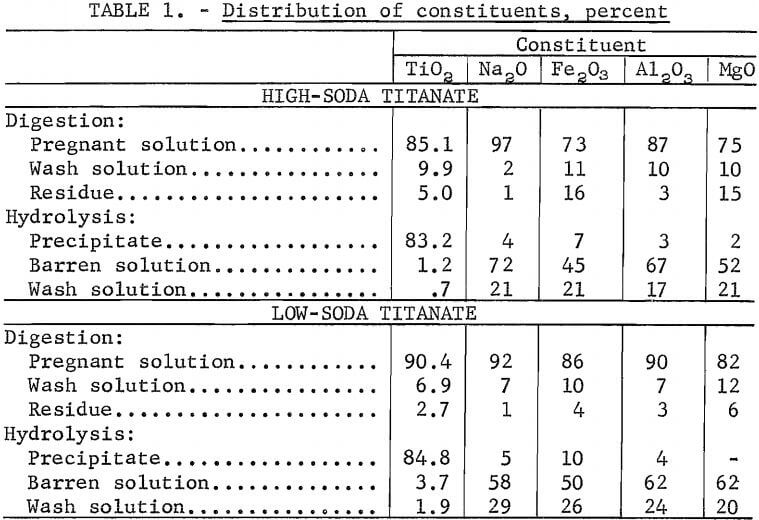
A laboratory procedure that simulated the sulfate process was used to evaluate HST and LST as feed materials. A flowsheet for the simulated sulfate process is shown in figure 3. The major difference between the laboratory procedure and the commercial sulfate process is that external heat was required for digesting the HST and LST in the laboratory procedure. When ilmenite is digested in the conventional sulfate process, heat resulting from the exothermic 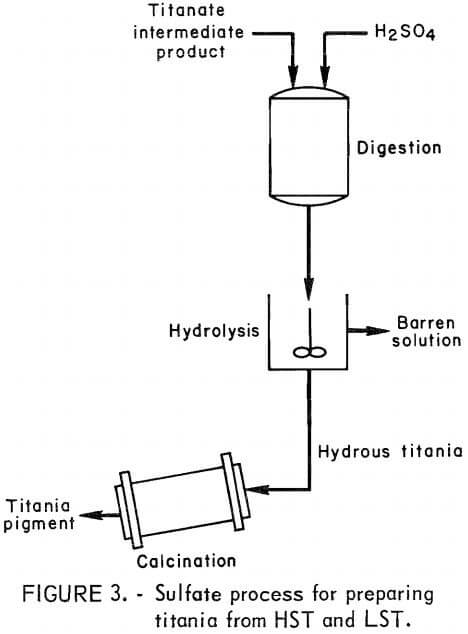 reaction between ilmenite and H2SO4 sustains the process. The laboratory procedure followed the sulfate process insofar as a precipitate was recovered by hydrolysis. The preparation of titania pigment from the hydrolyzed precipitate involves proprietary techniques developed by pigment producers that were unavailable for use in this investigation.
reaction between ilmenite and H2SO4 sustains the process. The laboratory procedure followed the sulfate process insofar as a precipitate was recovered by hydrolysis. The preparation of titania pigment from the hydrolyzed precipitate involves proprietary techniques developed by pigment producers that were unavailable for use in this investigation.
Using Hst and Lst as Feed Materials
Laboratory Procedures
Laboratory tests for evaluating HST and LST were performed using the following procedures:
- Digest HST and LST with 1 part titanate and 2 parts H2SO4.
- Heat the mixture (to initiate the reaction) to 200°-220° C. Maintain this temperature for 2 hours to dry the slurry.
- Cool the resultant material, add 4 parts water, and heat the pulp to 60° C to solubilize the titanium.
- Filter the resultant slurry, and wash the residue with water.
- Hydrolyze the pregnant solution containing 160 to 200 g/l TiO2 (as compared with 180 to 200 g/l TiO2 in the commercial sulfate process) to yield a hydrous titanium product as follows: Neutralize a 5- to 10-pct fraction of the pregnant solution with ammonium hydroxide to yield a seeding slurry; add the seeding slurry to the remainder of the pregnant solution at 90° to 95° C; and after 4 hours, filter the precipitate and wash with water.
Results With HST and LST Feed Materials
The commercial sulfate process utilizes concentrated or nearly concentrated (90 wt pct) H2SO4 to digest ilmenite. During early phases of this investigation, the use of concentrated H2SO4 to digest HST and LST resulted in extraction of 95 pct of the TiO2; subsequent hydrolysis recovered 94 and 98 pct of the soluble TiO2 from the respective pregnant solutions.
Because of the effectiveness of concentrated H2SO4 for extracting TiO2, dilute H2SO4 solutions were investigated also for digestion of HST and LST. The use of dilute acid for digestion would facilitate acid regeneration because barren solutions that usually contain about 30-wt-pct H2SO4 could be concentrated to 50-wt-pct H2SO4 with much less energy than is required to concentrate to 90 wt-pct.
TiO2 extractions of 94 to 97 pct were obtained when digesting HST and LST, respectively, with 50-wt-pct H2SO4. TiO2 extraction of 80 pct or less was obtained with solutions containing less than 50-wt-pct H2SO4. A H2SO4: titanate weight ratio of 2:1 was used in these experiments—the same as was employed with concentrated acid, but because of lower acid strength, a greater volume was required.
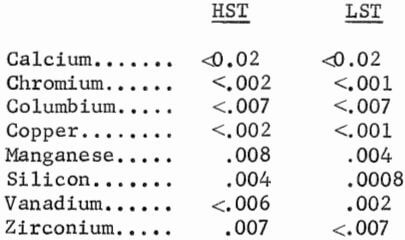
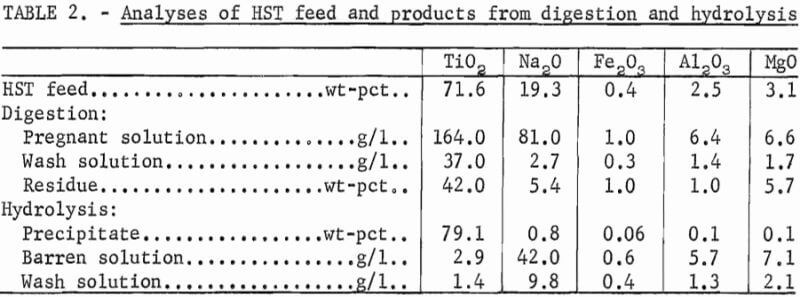
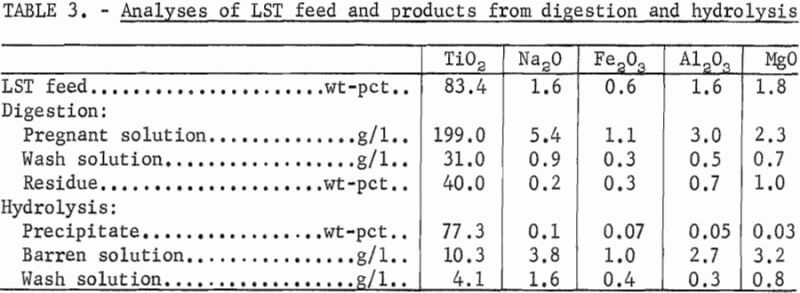
Table 1 shows the distribution of constituents in the pregnant, barren, and wash solutions, and TiO2 precipitates and residues resulting from treating the titanates. When treating HST and LST, TiO2 extractions were 95 and 97 pct, respectively. The resultant HST and LST pregnant solutions contained 85 and 90 pct of the TiO2, respectively. The remainder of the soluble TiO2 reported to the wash solution where it could be recycled and eventually recovered. The hydrous precipitate represented a TiO2 recovery of 83 and 85 pct from the HST and LST feed materials, respectively.
The analyses of pregnant, barren, and wash solutions, and the hydrolyzed products are shown in table 2 for treating HST and table 3 for treating LST. The TiO2 content was higher in the pregnant solution derived from treating LST, and the soda and other impurities were higher in the pregnant solution derived from treating HST. Other impurities shown by spectrographic analysis of the precipitates resulting from digestion and hydrolysis were as follows, in weight-percent:
Acid Regeneration
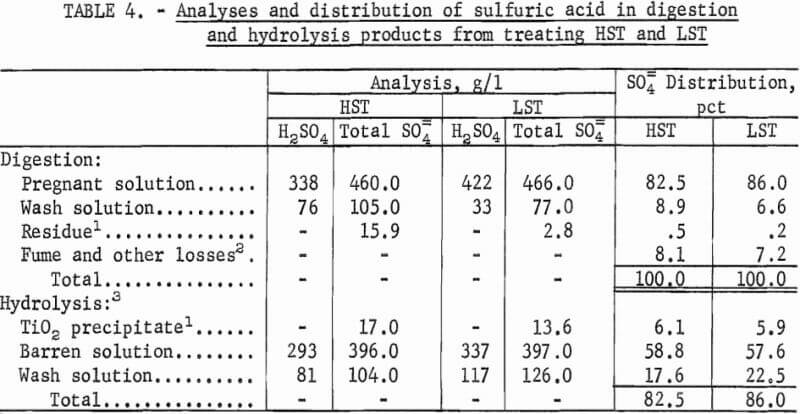
The analyses and distribution of H2SO4 in the digestion and hydrolysis products are shown in table 4. Acid solutions that are suitable for regeneration are the barren solutions that contain about 58 pct of the total H2SO4 used and have a total SO4 content of about 397 g/l (32-wt-pct H2SO4). Wash solutions from both digestion and hydrolysis contain about 28 pet of the total H2SO4 used.
These wash solutions with as low as 77 g/l total SO4 content also could be recycled to the process. Including both barren and wash solutions, about 86 pct of the H2SO4 used in treating HST or LST would eventually be available for regeneration. About 8 pct of the H2SO4 used is lost as fumes during digestion another 6 pct is lost by coprecipitation as sulfates during hydrolysis.
Electrodialysis appeared to be the most effective method for regenerating the barren acid solution resulting from treating HST because the solution contained sodium equivalent to 42 g/l soda. Electrodialysis not only reconcentrated the acid but also removed cation impurities such as sodium. The sodium content of the barren solution derived from treating LST was equivalent to only 4 g/l soda; therefore, a thermal-vacuum evaporation technique appeared to be the preferred method for reconstituting this acid solution.
The following assumptions were made in establishing a goal for regenerating acid from the sulfate process barren solutions:
- Fifty-wt-pct H2SO4 would be used for digestion.
- About 80 pct of the acid for digestion would be provided by regeneration.
- About 20 pct of the acid required would be provided by makeup of concentrated H2SO4.
- The barren solutions would be reconcentrated to at least 40-wt-pct H2SO4.
Work on acid regeneration by electrodialysis was performed by the Department of Chemical and Metallurgical Engineering, University of Nevada, Reno, Nev., under a research grant sponsored by the Bureau of Mines.
Barren solutions obtained from treating HST were upgraded from 312 to 680 g/l H2SO4 (26 to 49 wt-pct). The sodium content of the regenerated acid was equivalent to 3 g/l soda and should be compared with the 42 g/l contained in the barren solution. No more than 19 pct of the cation impurities such as Na, Al, and Mg present in the barren solution was carried over to the reconcentrated acid solution. Analyses of the barren and the regenerated acid solutions are given in table 5.
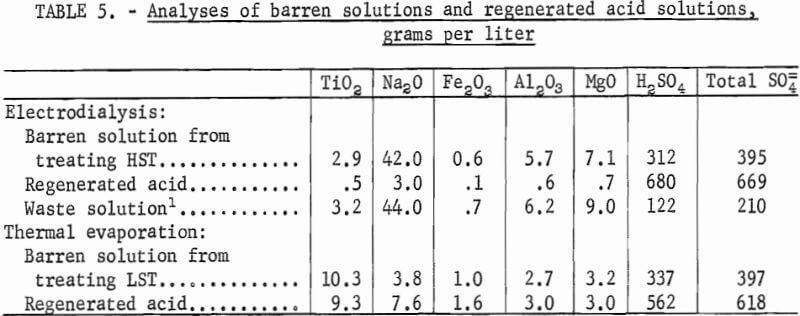
Acid regenerated from the barren solution from treating LST by vacuum-thermal evaporation contained about 562 g/l H2SO4 (42 wt-pct). Acid regenerated by vacuum-thermal evaporation had a considerably higher concentration of total sulfates than sulfate present as H2SO4; this is due to the increased concentration of Na, Mg, and other cations present in the regenerated acid. Laboratory tests indicated that the energy requirement for acid regeneration by electrodialysis was at least twice that required for vacuum-thermal evaporation. However, neither regeneration method, appeared economically feasible when compared with the cost of using virgin H2SO4.
Use of Regenerated Acid
Regenerated acid with the necessary makeup of concentrated H2SO4 to yield 50-wt-pct H2SO4 was used to treat additional LST and HST. A TiO2 extraction of 91 pct resulted when LST was digested with the acid regenerated by vacuum-thermal evaporation. The resultant pregnant solution was hydrolyzed to yield a precipitate containing 98 pct of the soluble TiO2 or 89 pct of the total TiO2. Analysis of the pregnant solution is shown in table 6.
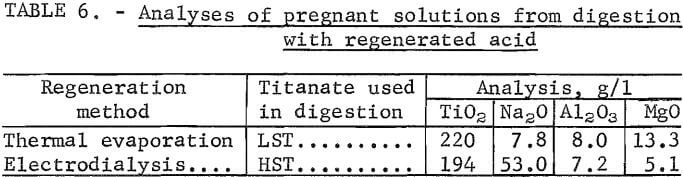
The acid regenerated by electrodialysis was used to digest additional HST. The TiO2 extraction was 90 pct. Precipitates resulting from hydrolysis contained 93 pct of the soluble TiO2 or 83 pct of the total TiO2. Analysis of the pregnant solution is shown in table 6.
The hydrous titania precipitated during hydrolysis of pregnant solutions originating from digesting HST or LST contained an average of 86 pct TiO2, 9 pct SO4, and had the following metallic impurities, in weight-percent:
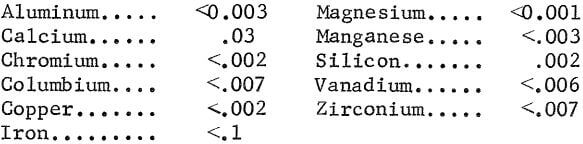
Analyses showed no significant increase in impurities in the titania prepared with regenerated acid, as compared with titania prepared from fresh acid.
Conclusions
This investigation has shown that HST and LST can be utilized effectively as a substitute for an ilmenite feed in the sulfate process for preparing titania. Digestion with 50-wt-pct H2SO4 resulted in extracting over 95 pct of the available TiO2 from these titanates. The resulting pregnant solutions containing 164 to 200 g/l TiO2 can be hydrolyzed to yield a high-purity titania precipitate, which represents about 95 pct of the TiO2 in the pregnant solutions.
Barren H2SO4 solutions that result when HST is digested with strong H2SO4 can be reconcentrated by electrodialysis and those that result when LST is digested can be reconcentrated by vacuum-thermal evaporation. Regenerated acid can be reused in digesting additional titanate to prepare titania without a significant increase of impurities in the product. Both acid regeneration methods studied in this investigation are energy intensive, and the use of regenerated acid appears uneconomical when compared with the cost of employing virgin H2SO4.
The Federal Bureau of Mines, as part of its goal to minimize undesirable environmental impacts associated with mineral processing, has investigated a soda-smelting technique for processing ilmenite into pig iron and a titanium slag that is suitable for a feed to a sulfate-process titania pigment plant. By this procedure, the generation of ferrous sulfate waste and its associated disposal problem is obviated. Molten titania slag was quenched, water leached, and filtered to yield a sodium titanate intermediate product containing about 70 wt-pct TiO2, 1 wt-pct Fe, and 20 wt-pct Na2O. This intermediate product, high-soda titanate (HST), was mixed with H2SO4, calcined, quenched, and water leached to produce a low-soda titanate (LST) product that contained only 2 wt-pct Na2O. Both the HST and LST were evaluated as feed materials for preparing titania by the sulfate process.
About 95 pct of the TiO2 was extracted from HST and LST by digestion in concentrated or 50-wt-pct-H2SO4 solutions. About 95 pct of the solubilized TiO2 was subsequently recovered from the titanyl sulfate solutions by hydrolysis. Barren solutions from hydrolysis, containing about 26-wt-pct H2SO4, were upgraded by vacuum-thermal evaporation or electrodialysis. The regenerated acid solutions were used to digest additional HST and LST.
