Anton Eilers, who was then interested in the lead smelting and refining business near Salt Lake City, Utah, made a somewhat radical departure from the regular practice at that time, which was to use but little lime in the slag, with a high percentage of iron. Lime was not only a cheaper flux than iron, but it enabled the metallurgist to make a more siliceous slag, an economical advantage in smelting where the smelting companies had to purchase either iron or lime to neutralize the excess silica, and penalize the ore shippers accordingly. The larger amount of both lime and silica in the slag also made it of lighter specific gravity, and a better separation of the metals and mattes was secured. This change in the formation of slags for lead smelting was brought about by Anton Eilers, prior to the Leadville mining excitement. We must also give Mr. Eilers credit for the general introduction of the hollow water-cooled cast-iron jacket for the blast furnace.
Again referring to the use of more lime and less iron in the formation of slags, there is no doubt but that it prevents the formation of a great many so-called “sows” in the lead crucible, such as were made in the early history of lead smelting in the Rocky Mountains; these sows consisted of lead, iron, arsenic, sulphur, copper, and other metals, including gold and silver, mixed with unconsumed fuel, and often, after vain attempts to cut them up or melt them to a suitable size for handling, were buried in the slag dumps to get them out of sight.
Another advantage derived from the adoption of high-lime slags when smelting ore containing arsenic is the production of less speiss (a by product heavier than matte, resulting from careless operation of the furnace, in the lead being driven out of the crucible and sows built up). This speiss, while usually not as rich in precious metals as matte made at the same time, is too valuable to be thrown away; but it is difficult to treat, and it was thrown away or piled on the slag dumps in the pioneer days of lead smelting in this country.
About the time of the introduction of the lower iron and higher lime slags in lead smelting, sulphide ore’s were encountered in Utah and Colorado. Matte was made as a byproduct in smelting and arsenic gave less trouble. The general supposition was that the arsenic was taken up by the matte and finally lost in the treatment of the matte. No doubt this was true to a great extent and I gave the matte the credit of taking care of the arsenic until I had to do some special lead smelting in Nevada. The ore to be smelted contained an average of about 3½ per cent, lead, 6½ per cent, iron, 2½, per cent, manganese, 7 per cent, zinc, 21 per cent, lime, 11.7 per cent, magnesia, 8½ per cent, silica, 1 per cent, alumina, 3 per cent, arsenic, and only a trace of sulphur, together with a very small amount of ore, which we called our lead ore, from another mine averaging 11½ per cent, lead, 20 per cent, iron, 4 per cent, zinc, 3 per cent, lime, no manganese, no magnesia, 30 per cent, silica, and only a trace of either arsenic or sulphur. From this it can be seen that there was more than enough arsenic to make a large proportion of speiss (especially as we smelted regularly considerable speiss containing gold and silver from some old slag dumps in the neighborhood), and no sulphur to make a matte to absorb it. At the same time, the bulk of the ore being so low in iron and manganese and high in lime and magnesia, I was compelled to make a slag very rich in lime and magnesia. The result was that we produced no speiss or matte, excepting once for a few hours when we fed in some sulphide ore to see what the results would be. During this smelting campaign of a little over one month, we had no trouble except with machinery and from shortage of fuel, which forced us to bank the furnace at one time for 24 hr. The result was $36,000 worth of argentiferous lead bullion made in a short smelting campaign without a single “freeze up” or “blow out” and no speiss product. The only detail in our practice which differed from the ordinary method of smelting was to cupel our bullion for shipment until it was rich in gold and silver, so as to use the lead as litharge over again in the charge. The fact that we were able to get rid of the arsenic in the ore and speiss from old slag dumps and make a comparatively clean slag led me to believe that the high percentage of lime base in the charge and the lack of iron with which the arsenic could combine, resulted in the volatilization of the arsenic, this action no doubt being assisted by the oxidizing influence of the litharge used. While our gold and silver losses were satisfactorily low, our lead losses were very high, which was to be expected when using the lead over and over again in such an oxidized mixture and keeping the coke consumption as low as possible. Coke was the only reducing agent used.
While making matte in the blast furnace in New Mexico and Arizona from low-grade copper ore containing plenty of sulphur and sometimes as much as 4 per cent, arsenic in the charge, no arsenic was found in the matte, either by our chemist or the purchaser, as it had all been volatilized.
Returning to the subject of lead smelting, I do not know of any important improvement in the composition of slags since lime slags were introduced by Mr. Eilers.
As the oxidized lead ores of the Rocky Mountain region became exhausted and “sulphides” were mined from the deeper mines, both silver and lead losses became greater, as no provision had been made for the proper separation of the matte from the slag at that time. For several years the slag was tapped into the ordinary small slag pots. The losses were very high by reason of the fact that much of the matte was mechanically held in suspension in the slag, the small pots and the quick cooling of the slag preventing proper settling. Further loss was caused by carelessness on the part of the furnacemen, slag being dumped without having been cooled and broken up to save the matte. Then came the introduction of square cast-iron settling boxes on wheels, which are still in use, but much larger and better constructed than at first.
The production of matte containing a little copper, if allowed to settle from the slag properly, produces a slag lower in value; at the same time the matte fall, in addition to the lead fall, increases the smelting capacity of the blast furnace, and requires a lower percentage of fuel. The smelting capacity of the lead blast furnace was also increased, sometimes doubled, by increasing the depth of the ore mixture in the furnace and increasing the blast pressure. This increase in depth and blast pressure was not practical as long as the metallurgist held to the idea that part of his fuel must be charcoal, as this would crush and pack under such a heavy burden, the fine charcoal and high-presssure blast causing “over” or “top” fires in the furnaces, increasing losses by volatilization and burning the fuel before it reached the proper melting zone of the furnace.
Improvements have also been made in the more economical handling of the ore, permissible only when handling large amounts, such as belt conveyors from crushers to storage bins, automatic dumping cars for feeding the charges into the furnaces, etc.
As the amount of sulphide ore to be smelted in the lead furnace increased, the matte settlers were improved. The sulphide ore was also ground fine and roasted before smelting. The fine roasted ore was found objectionable, causing too much flue dust and packing of the charge in the blast furnace. Briquetting was tried, but was found unsatisfactory and expensive. Then what was known as the Omaha & Grant roasting furnace was designed to roast and then fuse the fine ore into a pasty mass in the same furnace. This made an ideal product for the blast furnace when broken up properly. This method was used for years, but the loss in lead was excessive. The next and one of the most important advances in lead smelting was the introduction of the Huntington-Heberlein process, or “pot roasting,” which reduced the cost of roasting and the lead loss, at the same time producing a suitable product for smelting in the blast furnace. Following this improvement in the metallurgy of lead came the Dwight-Lloyd sintering machine, a cheaper method of doing the same work.
I think I have mentioned the most important improvements in lead smelting for the production of bullion containing gold and silver. Lead as a collector of gold and silver from very rich oxidized ore has its advantages; the loss of gold and silver in the refining of the lead bullion is smaller than in the refining of copper matte, and when the ore contains sufficient lead it is the only process to adopt. Lead smelting requires not less than 12½ per cent, lead in the charge, so that the concentration cannot be greater than eight of ore to one of bullion. If sulphides are to be smelted, the ore requires a comparatively sweet roast, and in the blast furnace three to five times the fuel is required that is necessary in the production of copper matte. In the blast furnace using ores containing copper sulphides as a collector less fuel is required, roasting is not necessary, more siliceous slags can be made, and a much higher concentration is possible by reason of the fact that a much lower percentage of copper is required as a collector. The preliminary removal of the zinc from a lead-zinc ore permits of an improvement in lead metallurgy. This subject has been quite fully covered in a previous paper by me and in articles by F. L. Wilson and others.
The methods of sampling ores today are practically the same as those used more than 30 years ago. At large copper-smelting plants they have adopted automatic samplers to cut expense where the ore not only comes from their own mines, but is comparatively low grade. In custom plants where small lots of extremely rich ore are sampled and followed by samples taken from large lots of low-grade ore, automatic sampling machinery is not reliable. Recently I had an opportunity to observe the sampling of a large lot of rich ore at the Selby Smelting & Lead Co.’s works and I could not ask for a better system of sampling. I will not attempt to describe the method here except to say that it was not done automatically and was expensive, but absolutely fair to both the shipper and the company.
I have not touched on the subject of lead refining for the reason that in the West we are more interested in the production of lead bullion containing the precious metals. The bullion can be shipped to refiners in the East to better advantage than to refine it here and ship the metals separately.
In conclusion I wish to call attention to the following items in this article:
- The advantage of high-lime and low-iron slags, especially when smelting lead ore containing arsenic.
- The advantage of enriching the lead bullion by cupelling so as to use the lead over again as a collector of the precious metals; also the saving in freight on lead in isolated districts where the freight costs amount to nearly as much as the lead is worth.
The cupelling furnace referred to is such as I used in Leadville, Colo., and in Nevada, large enough to cupel 8 tons of bullion in 24 hr. It would also be suitable for the saving of antimony, thus shipping cleaner lead.
While the advantages in the use of high-lime siliceous slags in lead smelting which Mr. Bretherton discusses in this paper are obvious under certain economical conditions, he gives me credit for the introduction of such slags when it does not belong to me. It is true I did considerable experimental work, off and on, for the purpose of finding such slags, while at the Germania in 1877 and 1878, but I did not have occasion to introduce any such slags for regular work, because I had no ores which fitted the economical conditions for using high silica and high lime, except in isolated cases for very brief periods. On the contrary, the ores I had to treat were mostly very ferruginous and very low in silica, so that, economically, I was compelled to run nearly always on slags, which allowed me to use the abundant flux I had without cost.
It was August Raht who first had occasion to establish and use for long periods a slag high in silica and lime, and low in iron. This was when he was confronted with the task, of smelting the Horn-Silver ores at the smelting works at Franklin, Utah, with as little expenditure for fluxing material as possible. I was at that time in Leadville, Colo., where, in the new works I had built, the Billings and Eilers smelters, I had occasion to use profitably many slags, but never anything as high in the earthy bases and in silica as Mr. Raht had to use.
On Dec. 4, 1881, Mr. Raht sent me the following two analyses of slags, taken from the regular run on different days, and the remarks accompanying:
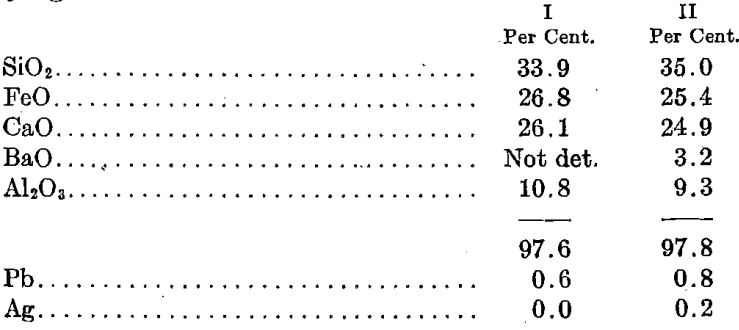
I. Produced with coke = 15.2 per cent.
Bullion 163 oz.; lead contents of charge, 18.7 per cent.
Blast pressure, 5½ oz. per square inch.
II. Produced with coke = 14 per cent.
Bullion 152 oz.; lead contents of charge, 20.1 per cent.
Blast pressure, 6¾ oz. per square inch.
This was a remarkably clean slag and, according to Mr. Raht, kept the furnaces in excellent condition and allowed of good tonnages. I looked up the nearest chemical formula that I could derive from the above percentages and found the following:
3FeO.2SiO2 + 4CaO.2SiO2
Assuming that the slag consisted only of silica, iron protoxide, and calcium oxide, it gives the following percentages:

and assuming that 12 per cent, of iron and lime was replaced by foreign bases (in this case principally Al2O3 as given by Mr. Raht) the following percentages result:
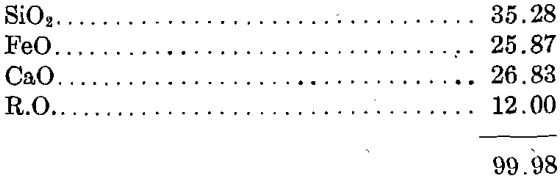
I therefore took this slag of Mr. Raht’s to be a “correct slag” with all the virtues that correct slags have.
It will be noticed that it is a singulo-silicate of iron and lime plus a bisilicate of iron.
I occasionally used this slag, later in life, with advantage in both lead and copper smelting.
Mr. Bretherton also kindly credits me with the introduction of cast- iron water jackets. Cast-iron water jackets closed on top with an outlet pipe for the hot water on the side near the top (the inlet pipe for the cold water being near the bottom of the jacket) were in use at a few smelting works when I came to Salt Lake City, but frequent burning out on account of steam spaces forming, made their use rather costly. Together, Augustus Steitz, of St. Louis, and I devised a jacket with an open neck 7 in. high, of box shape, on the top of the jacket, into which the cold-water pipe reached down 10 or 12 in. and the hot-water outlet pipe was very near the top of the neck, thus preventing any steam spaces in the jacket. It is true this jacket was generally introduced where cast- iron jackets were used. Some people, however, used costly wrought-iron jackets in preference, the reason for which has never been apparent to me.
The advantages which Mr. Bretherton sets forth, derived from the use of high SiO2 and high CaO slags in general, coincide with my own observations. Of course, the main reason for using such slags must always be the economic side in the selection of flux, but the other incidental advantages which he mentions are also of great moment. The elimination of arsenic in certain charges can, according to my experience, be increased by cutting the fuel percentage slightly below that needed for perfect reduction, and by giving, at the same time, say, 1 per cent, more CaO than the formula requires. This, however, may increase the silver loss slightly.
