Table of Contents
The Bureau of Mines once modified a commercial atomic absorption spectrophotometer to rapidly determine trace levels of mercury by cold-vapor atomic absorption spectrophotometry AA. Such diverse materials as ores, mill products, stream water, sediments, and flue dust have been analyzed. A very high dynamic range of 0.2 ng/ml to 1 µg/ml of mercury in solutions is routinely analyzed by this instrument.
Mercury in the environment may have serious toxicological effects on plant and animal life, even at the parts per billion level. The demand for analyses of many environmental samples has created a need for a simple and rapid method for the routine determination of mercury at this low level.
Many analytical procedures have been published for the determination of mercury in both natural and manmade environmental materials. One of the earliest was a classical colorimetric technique. Other approaches superseded colorimetry: chromatography for methyl mercuric chloride in water, neutron activation for mercury in solid materials, and flameless atomic absorption spectrophotometry.
Neutron activation requires that a solid sample be sealed in quartz ampoules. Although the procedure is inconvenient and expensive, the materials can be analyzed without pretreatment. The method most sensitive for mercury analysis is flameless atomic absorption spectrophotometry, using either the graphite furnace or the cold-vapor method. In practice the cold-vapor method has proven to be the most reliable, and numerous studies in the improvement of the technique have been made. Some of these studies include
- The work of Hawley and Ingle, which gives a more fundamental understanding of the variables that affect the procedure.
- The work of Simpson and Nickless, which describes a rapid dual channel instrument.
- A paper by Tong, which describes a stationary absorption cell method for the determination of mercury where mercury is reduced and partitioned between an aqueous and gas phase and where measurement is made by passing the mercury resonant light beam through the gas phase.
- A paper by Tuncel and Ataman, describing a cell they designed with the same profile as the light beam from the hollow cathode lamp and which increases sensitivity.
The Bureau’s Albany Research Center has constructed an analytical system for the cold-vapor determination of mercury. The basic component is a modified Perkin-Elmer model 303 atomic absorption spectrophotometer. A special reduction cell and a combined vaporization chamber and optical cell have also been fabricated. The vaporization chamber is heated to 600° C, and the optical cell is heated to 150° C (surface temperature) to prevent water and mercury condensation in the system. The modification of an atomic absorption spectrophotometer described here can be used as a guide in constructing a similar system in other analytical laboratories. Older atomic absorption spectrophotometers, which are seldom used because of the technical superiority of newer instruments, can be dedicated to the determination of mercury.
Experimental
Equipment
- Perkin-Elmer model 303 atomic absorption spectrophotometer equipped with an automatic null recorder readout and Sargent recorder, model SR, with a 10-mv-range plug.
- Mercury hollow cathode lamp.
- Two variable autotransformers (110 v).
- 5-inch tubular burner head or similar device.
- Low-capacity vacuum pump.
- Flow meter with a 10- to 1,900-ml/min air range.
- Millivoltmeter calibrated to read 0° to 300° C using an iron-constantan thermocouple.
- Iron-constantan thermocouple.
- High-purity nitrogen gas.
Reagents and Standards
All standard solutions should be stored in glass containers rather than plastic containers because glass absorbs mercury from dilute solution less readily than plastic. All solutions are prepared from reagent-grade chemicals. Glassware used for the more dilute solutions (1 µg/ml or less) should be soaked in 50 pct HNO3 overnight and washed with mercury-free distilled water.
A master standard solution is prepared by weighing 1.3535 g HgCl2 into a 1-liter volumetric flask, adding 50 ml concentrated HNO3 and diluting to the mark with distilled water (1,000 µg/ml). By appropriate dilution of the master standard, working standards are prepared daily in the usual working range of 0.01 µg/ml – 0.1 µg/ml. The working standards are preserved with K2Cr2O7 + HNO3 made up to a final concentration of 0.1-molar K2Cr2O7 and 2 vol-pct HNO3.
Reductant solution is prepared by weighing 1 g SnCl2 into a beaker and adding 1 ml concentrated HCl and enough distilled water to adjust the volume to 100 ml. The reductant solution should be freshly prepared before analysis.
Fabrication and Modification of Equipment
The spectrophotometer was modifed by removing the gas connections, the nebulizer assembly with the exception of the burner chamber, and the rear guide rod for the burner chamber. The guide rod was replaced with a 108-mm-long rod of the same diameter, so the optical cell could be raised to the light path without excessive free motion.
The reduction cell was fabricated by our glass blower and is illustrated in figure 1. The cell body is made of standard Pyrex tubing, 14 mm OD; the side arm and bottom tube are made of standard 7-mm-OD Pyrex tubing; and the frit used is extra coarse.
The optical cell and vaporization chamber are illustrated in figure 2. The optical cell is made of standard Pyrex tubing, 20 mm OD with 21-mm-square quartz windows fastened to the cell with epoxy cement. (The quartz windows were cut from the end windows of worn-out hollow cathode lamps.) The reduction cell, vaporization chamber, and optical cell were all fabricated in this laboratory.
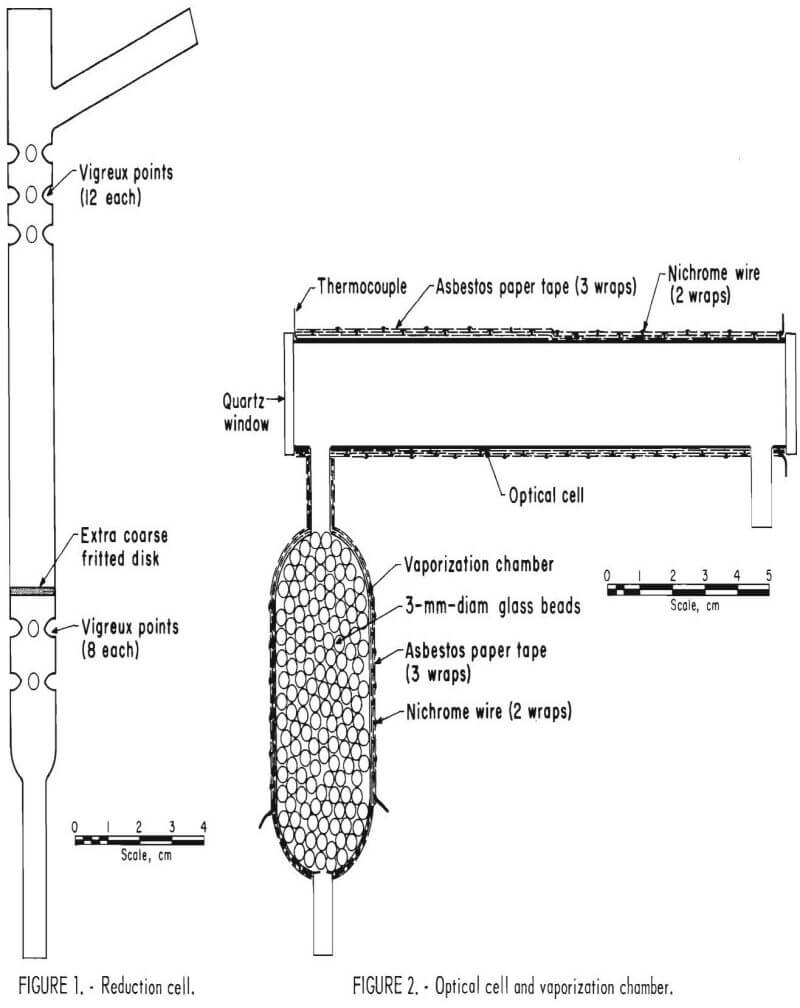
After placing an iron-constantan thermocouple on the optical cell, the optical cell and the vaporization chamber were both wrapped with one layer of asbestos paper that was treated with a 40-pct solution of sodium metasilicate. After the sodium metasilicate was dry, the optical cell was wrapped over its entire length with 14 turns of No. 28 nichrome wire, making sure the wires did not touch one another. The nichrome wire was held in place while another layer of sodium metasilicate treated asbestos paper was wrapped over the nichrome wire. The nichrome wire was then wrapped back upon itself (14 turns for a total of 28 turns). The wire was held in place and wrapped over with the sodium metasilicate treated asbestos paper. The vaporization bulb was similarly wrapped (7 turns of No. 26 nichrome wire for the length of the greatest diameter of the bulb for a total of 14 turns). Approximately 1 inch of wire was left protruding (both leads) to connect to the source of power. These leads were covered with fiberglass-wrapped silicone rubber insulation. The optical cell and vaporization chamber were placed in a drying oven at 100° C overnight so the sodium metasilicate, asbestos, and the epoxy cement would cure. The support for the optical cell was fabricated from a 5-inch tubular burner head by cutting the tube in half on a milling machine. (See fig. 3.)
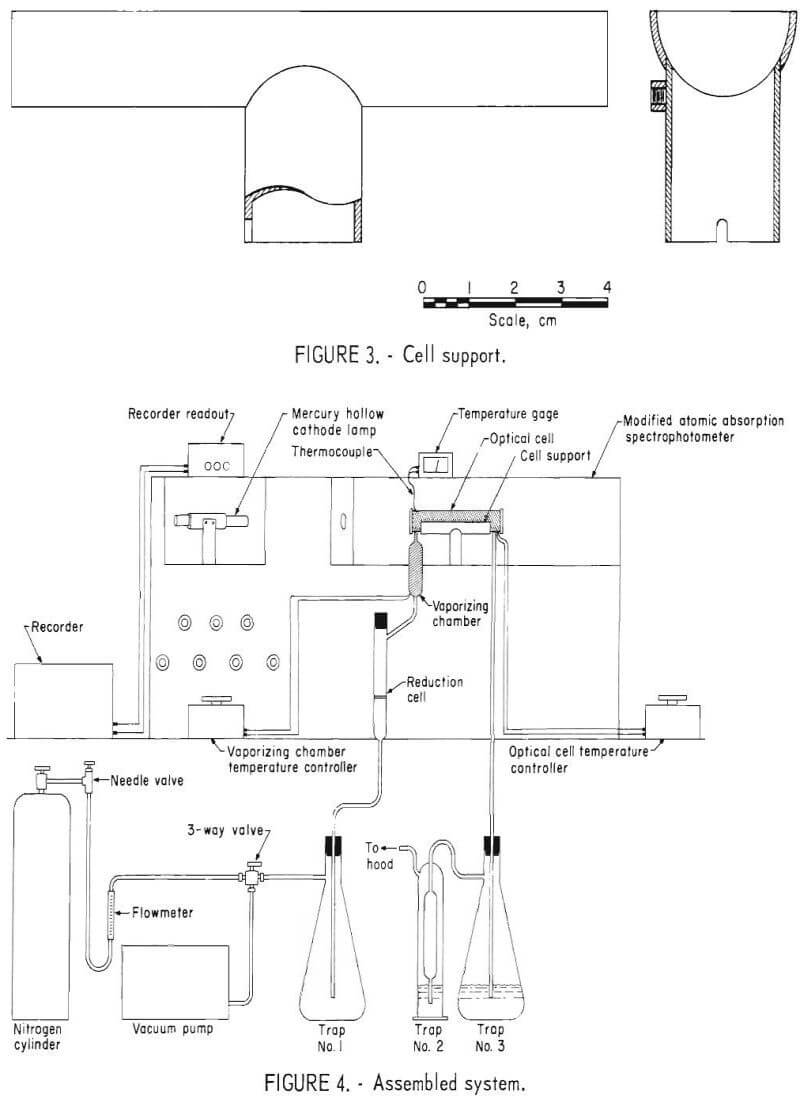
Assembly of the System
The system was assembled as illustrated in figure 4. Some of the important points of the assembly which may not be clear in the illustration are listed below:
- All connections were made with polyvinyl chloride tubing.
- A three-way valve was used to shift quickly between the nitrogen sparge mode and the vacuum mode.
- A 1-liter vacuum flask was installed in the line to trap spent sample and wash solutions (Trap 1).
- The reduction cell, mounted vertically, was closed with a No. 00 rubber stopper.
- To stabilize the optical path, the optical cell was held to the cell support with steel springs.
- Mercury vapors emmitted from the samples and standards were absorbed in two chromic acid traps placed in series at the end of the system (Traps 2 and 3).
Sample Preparation
Before sampling, all sample containers should be soaked in 50 pct HNO3 overnight, washed with mercury-free distilled water, dried and protected from environmental contact until the sample is obtained. Liquid samples should be preserved by the method suggested by Avotins and Jenne. Before the sample is taken, enough purified HNO3 and K2Cr2O7 are introduced into the sample container so that the final concentration is 2 pct HNO3 and 0.1-molar K2Cr2O7.
Samples introduced into the system must be liquid. Solid samples such as ores, slags, and soils are converted to solutions, generally by a wet digestion method such as the method of Malaiyandi and Barrette or a modification of this method.
Instrument Adjustment
The chart recorder parameters are as follows. Use the 10-mv range plug, the range attenuator in the off position, and the filter in the No. 1 position. On the recorder readout, the noise suppression is never greater than 2, and the scale expansion used is never greater than a factor of ten. Establish the nitrogen gas flow at 270 ml/min on the calibrated flow meter. Adjust the autotransformers for both the optical cell and the vaporizing chamber so that the temperature for the optical cell is 150° C±10° C as read on the temperature gage and the temperature for the vaporizing chamber is 600° C (just under a red heat as seen by the exposed nichrome wire). The following atomic absorption instrumental conditions are used: 0.7-nm spectral bandpass, ultraviolet range, 253.7-nm wavelength, and 5-ma lamp current. Aline the hollow cathode lamp and raise the optical cell into the light path, adjusting the mercury cell for maximum mercury signal. Allow the system to reach equilibrium as evidenced by a steady recorder baseline.
Analysis Procedure
- Remove rubber stopper and pipet 300 µl of 1 pct SnCl2 into the reduction cell.
- Replace the rubber stopper, switch the recorder chart drive on, and adjust the baseline to zero.
- Remove the rubber stopper and pipet 1 ml of sample solution into the reduction cell. Quickly replace the rubber stopper.
- Allow the reduced mercury vapor to flow through the system. The usual time elapsed from injection of sample to scribing of the peak and return to baseline is -25 seconds.
- After the recorder has returned to the baseline, remove the stopper and switch the reduction cell from nitrogen sparge mode to vacuum mode. IMPORTANT: If the stopper is not removed before switching to the vacuum mode, chromic acid will be aspirated back through the system. Allow the contents of the cell to flow into the trap (Trap 1). Wash the reduction cell with mercury- free distilled water, and allow the washing to flow into the trap.
- Reposition the three-way valve into the nitrogen sparging mode and introduce another sample for analysis, as in steps 1 through 6.
Results
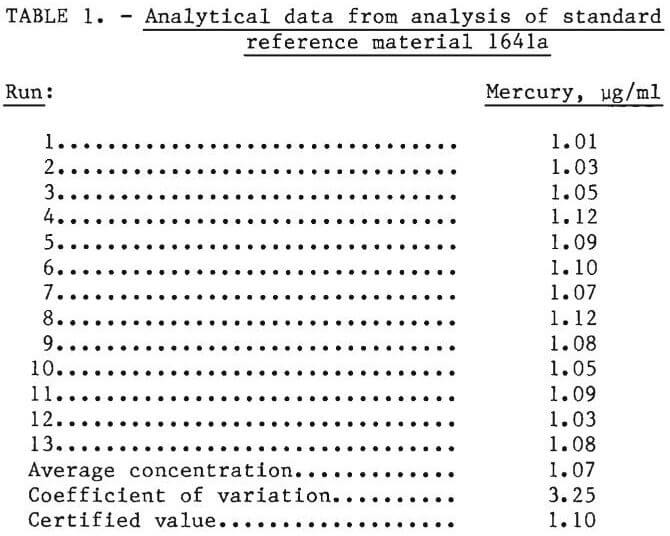
To determine the accuracy and repeatability of the cold vapor method, using this apparatus, standard reference material 1641a (mercury in water), supplied by the National Bureau of Standards, was analyzed. This standard reference material is preserved with nitric acid and a trace of gold.
The reference material was analyzed 13 times for mercury. The results were tabulated; average concentration and the coefficient of variation, v, was calculated as follows:
v = (100/X)√∑d²/(n-1)
where: X = average concentration, raicrograms per milliliter,
d = difference of the determination from the average concentration, and
n = the number of determinations.
Table 1 shows the results obtained, the average concentration, the coefficient of variation, and a comparison between certified results and results obtained by cold vapor atomic absorption.
Discussion and Conclusions
There are many commercially available mercury cold-vapor systems. Many of them use a much larger reduction cell than the system described in this paper. The greater volume causes a further dilution of the mercury vapor with the carrier gas and therefore lowers the sensitivity of the system. Other systems generally place a desiccant of anhydrous magnesium perchlorate between the reduction cell and the optical cell to remove water vapor. The removal of water vapor reduces light scattering in the optical cell and improves sensitivity. A disadvantage of using the magnesium perchlorate desiccant is that it is thought to absorb some mercury vapor and cause low and variable results. In the system presented here, a desiccant is not used because the heated vaporization chamber and optical cell prevent both water and mercury condensation.
For the past 2 years, this cold-vapor mercury analysis system has been successfully used at the Albany Research Center. It has been a more rapid and sensitive method for determining mercury than the colorimetric dithiazone or the flame atomic absorption methods previously used. The described system offers precise and accurate analysis as shown by the analytical data. The system is also rapid and offers good sensitivity for a working range of 0.2 ng/ml (200 ppt) to 1 pg/ml (1 ppm). This large dynamic concentration range generally allows for the direct injection of liquid samples into the system without a prior dilution or concentration of the sample.
An important result of this research is that the system has been dedicated to an obsolescent piece of atomic absorption equipment that would have been otherwise declared surplus. The cold-vapor system could also have been adapted to a more modern atomic absorption spectrophotometer without any further modification.
