Table of Contents
Research was conducted by the Bureau of Mines to determine the feasibility of using electrokinetic densification to dewater Bayer process red mud, magnetic black mud prepared by pressure digestion of red mud in the presence of ferrous sulfate, and magnetic black mud formed by simulated Bayer digestion of Jamaican bauxite with added ferrous sulfate. Tests showed that the solids content of presettled muds could be increased from 25 pct to approximately 40 to 48 pct by gravity draining followed by electrodewatering for approximately 48 h. Electrodewatering may not be practical because of increased reagent and processing costs and because the muds must be thoroughly washed prior to electrodewatering to remove dissolved ions and decrease mud conductivities.
A voluminous and troublesome red mud waste is generated when bauxite is leached with caustic to recover alumina by the Bayer process. The mud is composed primarily of fine grained iron oxides, in particular hematite and goethite. Because of its near colloidal nature, the mud is slow settling and difficult to wash. Current plant practice is to add flocculants to accelerate settling during the washing stages and to dispose of the mud in containment ponds. After years of settling, the mud contains 50 pct water and is thixotropic.
Methods investigated for consolidating and dewatering red muds include centri-fugation, flocculation, filtration, chemical treatment, and drying. Although these techniques increase the rate of dewatering, none, with the exception of drying, increase the ultimate settled density beyond that achieved in mud lakes. Drying adds considerably to the processing costs.
Many uses have been proposed for the red mud waste, but they depend on finding an economic process for dewatering the suspension. Recent Bureau research has focused on techniques to increase water reclamation for recycling and decrease water needs in hydrometallurgical processes by converting voluminous hydrous metal oxides into denser compounds, or by forming alternative compounds that filter and wash better. Research showed that the hematite and goethite in Bayer red mud could be converted to magnetite by reacting the mud with ferrous salts at 200° to 260° C in a pressure reactor. The mud slurry produced by this treatment was black in color and magnetic. It was also demonstrated that black magnetic mud could be formed without detrimental effects on alumina extraction by adding ferrous sulfate to a simulated Bayer caustic digestion of Jamaican bauxite.
Studies were initiated to determine the filtration and magnetic characteristics of the black mud. It was shown that black mud filtered at about the same rate as red mud, i.e., very slowly. The behavior of black mud in the presence of a magnetic field is currently being investigated.
This report summarizes the results of exploratory research into the application of electrodewatering methods to Bayer red mud, black mud prepared by pressure digestion of red mud in the presence of ferrous salts (converted mud), and black mud formed by simulated Bayer digestion of Jamaican bauxite in the presence of ferrous sulfate. The principal method investigated was electrokinetic densification, the application of a dc electrical field to a suspension of solid particles in a liquid, usually water. Electrokinetic densification combines the effects of electrophoresis and electro-osmosis with gravity filtration.
Electrophoresis is the migration of solid particles in the suspension under the influence of the applied electric field. Most soil particles are negatively charged and migrate toward the anode. To maintain an overall charge balance, the water near the particle surface contains an excess of positive ions and the charged water molecules migrate electro- osmotically toward the cathode. The theory of electrodewatering has been well documented.
Electrokinetic densification has been shown by the Bureau and others to be effective for consolidation of fine-particle slurries. Metal mine tailings, mine drainage sediment and coal waste, phosphatic clay waste, and clay slimes have been successfully dewatered by electrodewatering techniques.
A literature review indicated that Bayer mud possesses most of the properties necessary for good response to electrodewatering methods—small particle size (<10 µm), high zeta potential, high degree of dispersion, and high pH. However, because of the difficulty of washing the suspension, entrainment of caustic and aluminate salts causes the mud to exhibit high conductivity.
Electrokinetic dewatering works best on materials that have low specific conductivity values (<1,000 µmho·cm-¹). Low conductivity assures that the current in the electrodewatering cell is carried primarily by the charged particles and electro-osmotic movement of positively charged surface water and not by ions dissolved in the bulk water. Because conductivity can be decreased by removing dissolved ions, most electrodewatering tests were performed with well-washed mud samples.
Experimental Materials and Sample Preparation
Red mud from the underflow of the No. 8 mud washer at a Bayer alumina plant processing Jamaican bauxite was obtained. The mud had an average percent solids of 22. Although it had been processed through eight washing stages, the mud still exhibited a relatively high specific conductivity of 18,900 µmho·cm-¹ because of entrained caustic and sodium aluminate. In most cases, the mud was washed further before electrodewatering tests.
This red mud was converted to black mud by adding 100 pct of the stoichiometric amount of FeSO4·7H2O required to convert all of the hematite (Fe2O3) in the mud to magnetite (Fe2O3·FeO). The amount added gave a 2:1 Fe³+-Fe²+ ratio in the mixture. Sufficient 10N NaOH solution was added to give a pH of 12, and the mixture was heated for 1 to 5 h at 200° to 250° C in a stirred stainless steel pressure reactor.
In Bayer simulation studies, 200-g samples of minus 10-mesh Jamaican bauxite were mixed with 350 mL of 10N NaOH, 950 mL H2O, and variable quantities of FeSO4·7H2O. The ferrous sulfate addition ranged between 20 and 120 pct of the stoichiometric amount required to convert the ferric iron in the bauxite to magnetite:
H2O + Fe2O3 + FeSO4 → Fe2O3·FeO + H2SO4…………………………………………………….(A)
If no FeSO4·7H2O was added, a red mud product was obtained. The bauxite mixtures were heated for 1 h at 250° C in the stainless steel pressure reactor.
After the digestion or conversion reaction, the resultant slurries were allowed to settle and the pregnant liquor was removed. The mud products were washed by a settling and decantation procedure until a desired specific conductivity, generally 1,000 µmho·cm-¹, was obtained. Mud conductivity was measured with a dip-type conductivity cell. The washed muds were allowed to settle to approximately 25 pct solids before testing in the electrodewatering cell. Settling usually took 1 to 3 days and the settled muds, although thick, were still pourable.
Electrodewatering Test Apparatus
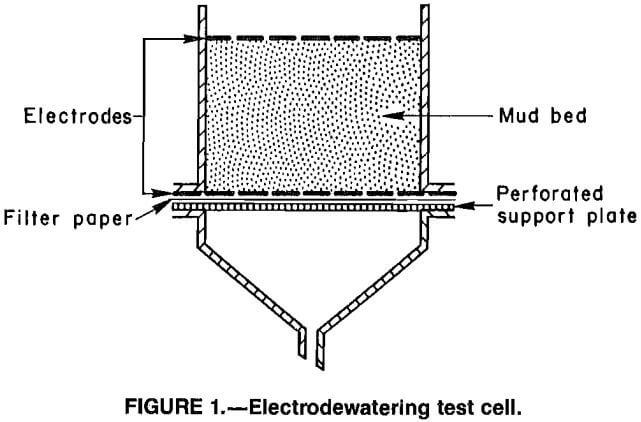
The test cell (fig. 1) was constructed from a 6.8-cm-ID cylindrical glass filter unit that had a 200-g mud capacity. The apparatus included two horizontal electrodes fabricated from mild steel wire mesh. The upper electrode was pressed into the surface of the mud. The lower electrode was supported by a flange between the cell body and a drainage funnel connected to a collection flask. A filter paper inserted between the lower electrode and a perforated support plate prevented solids from passing out of the cell but allowed free drainage of fluids.
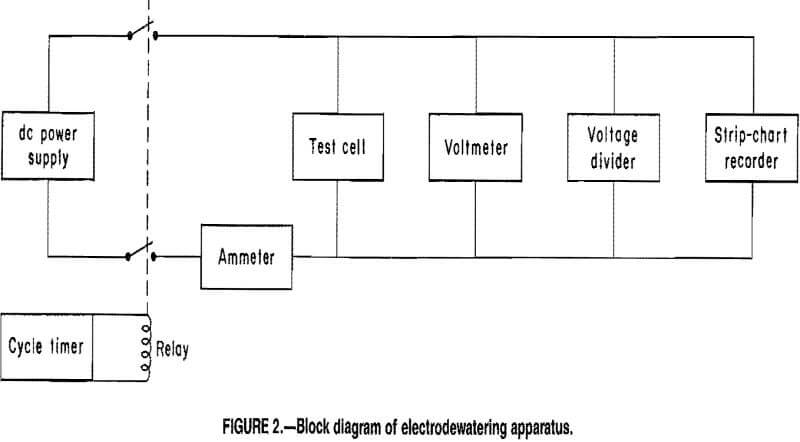
Power was furnished by a constant current dc power supply.
The system also included a cycle timer that either turned off the power to the cell or reversed the polarity for 6 min each half hour. The off and reversed current cycles allowed periodic depolarization of the electrodes. Previous researchers determined that periodic power reversal resulted in improved dewatering rates and increased densification of mill tailings compared with results from tests conducted with constant dc power application. Periodic power interruption also decreased power consumption, although somewhat longer times were required to achieve maximum dewatering. Cell voltage was continuously monitored with a strip-chart recorder wired in parallel with the cell electrodes. A voltage divider was used to expand the range of the recorder. Figure 2 shows a block diagram of the test system.
Methods
The percent solids in the mid samples was determined at the beginning and end of each experiment by measuring the weights of a well-mixed mud sample and the solids obtained after filtering, washing, and drying at 110° C. The measured solids content was determined by dividing the dry weight by the wet weight of the sample.
The test cell and collection vessel were weighed periodically during the course of the experiment and the average solids content of the rand in the cell could be calculated from the weight of mud at any given time, (t),
Weight solids in initial mud sample/(Weight of mud)t x 100 = (percent solids)t……………………….(1)
Values calculated from equation 1 were used to construct dewatering profiles, plots of the change of solids content in the mud as a function of time. As discussed later in the text, the calculated percent solids value is only an approximation and is most accurate during the early stages of the experiment. The mud was allowed to gravity drain until liquid flow stopped. This usually took 24 h, although most of the dewatering occurred during the first 6 to 8 h. Then power was applied to the cell and the experiment was conducted until filtrate stopped flowing. Since the upper electrode was imbedded in the surface of the mud, interelectrode spacing decreased over the course of the experiment as dewatering took place and the mud compacted.
Results
Preliminary Electrodewatering Tests
Preliminary experiments established operating procedures and techniques. Red mud and converted black mud were used for these tests. Variables studied included electrode polarity, cell current and voltage, duration of power application, mud conductivity, and pH.
The following observations were made from the preliminary tests:
- Best dewatering results were obtained when the cell was operated in the electrophoresis (EP) mode with the bottom electrode acting as the anode and the upper electrode (cathode) pressed into the surface of the mud. Tests were performed by continually cycling the power in the EP mode for 24 min following by 6-min off cycles. With this electrode configuration the positively charged anode attracted the negatively charged mud particles electrophoretically, and settling was aided by the effects of gravity. Hydrogen gas formed by electrochemical reduction of water at the cathode was vented to the atmosphere at the top of the cell. Cathode reaction:
2H2O + 2e- → H2 + 2OH-……………………………………………………(B)
Water from the mud percolated through the mud and exited the bottom of the cell. It was expected that operating the cell in the EP mode would segregate the mud into solid and liquid layers with the solids drifting downward toward the anode as the water was drawn upward toward the cathode. Liquid would then be siphoned off the top. This type of segregation was seldom observed, and when it did happen, drainage occurred after overnight operation. Apparently the consolidated mud solids were sufficiently permeable and the gravitational force exceeded the upward electro-osmotic force. Therefore, the liquid drained from the bottom of the cell at about the rate it formed.
- When the cell was operated with the opposite electrode polarity, in the so-called electro-osmotic (EO) mode, the lower electrode acted as the cathode, and the positively charged water molecules should have been drawn electro- osmotically through the mud bed to the bottom of the cell. In practice, hydrogen formed at the cathode surface and became entrapped in the viscous mud. The trapped gas created voids and sometimes lifted the whole mass of mud and destroyed the electrical continuity of the cell.
- Oxidation of iron caused corrosion of the anode. Since the corrosion products were hydrated iron oxides, the primary component of the red mud, there was no concern about introducing foreign material into the mud via corrosion. Anode reaction:
Fe° + nH2O → Fe(OH)n + 2nH+ + ne-……………………………………………………………(C)
- Operating the cell at excessively high current and voltage caused oxidation of water to compete with iron oxidation at the anode.
H2O +½O2 + 2H+ + 2e-…………………………………………………………………………(D)
A current of 40 mA (current density about 1.1 mA, per square centimeter of electrode grid area) gave adequate dewatering rates. At higher currents, gassing at both the anode and cathode became a severe problem, and at lower currents, electrodewatering rates decreased appreciably. The initial voltage was a function of slurry conductivity, and voltage increased during the experiment primarily because good electrical contact between the mud and the electrodes became more difficult as the mud thickened. Poor contact created an increased resistance in the contact zone, which in turn increased the voltage drop and decreased the current through the cell. By pressing the upper electrode into the mud, the contact improved and the resistance was lowered for a few power cycles. However, the improvement was only temporary and electrode contact problems plagued the later stages of most of the experiments.
- Reverse polarity tests (EP-EO) were conducted by alternating the electrode polarity between 24-min EP and 6-min EO cycles. These tests yielded the same dewatering rates as observed with the EP experiments in which the power was cycled on and off. The latter mode of operation was more energy efficient because the cell was operated without power for a portion of each cycle.
Because the preliminary tests indicated that operation of the cell in the EP mode gave the best dewatering results, all subsequent experiments were conducted with the cell wired in this configuration. Figure 3 depicts a typical set of EP dewatering curves. The middle line shows the accumulation of filtrate in the collection vessel and represents the effects of gravity draining and electrodewatering. The upper line represents the total weight loss of the mud in the cell and includes gravity draining, electrodewatering, and evaporation factors. The difference between the upper and middle lines is a measure of evaporative weight loss. The lower line shows the change in the solids content of the mud over the course of the experiment. The points on this curve were calculated using equation 1. The mud gravity drains to a constant solids concentration after
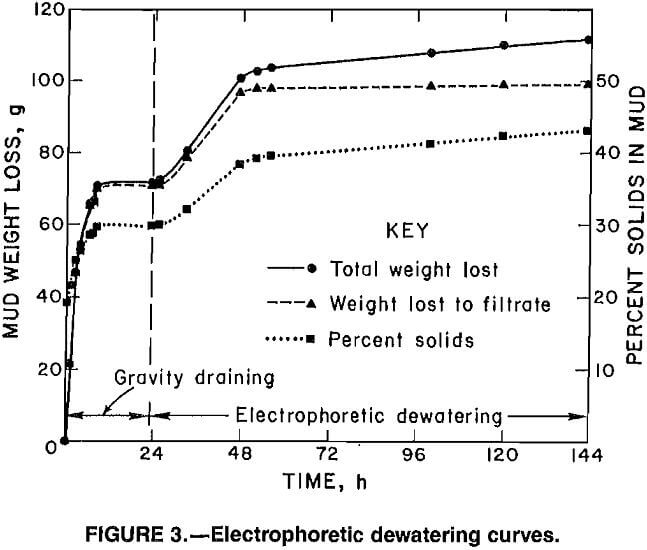
about 8 h, and application of power to the cell causes a significant increase in the dewatering rate. After 24 h of power the rate of filtrate accumulation decreases to almost nothing. However, the cell continues to lose water owing to evaporation because the voltage increases as the mud consolidates and the slurry temperature increases because of resistive heating.
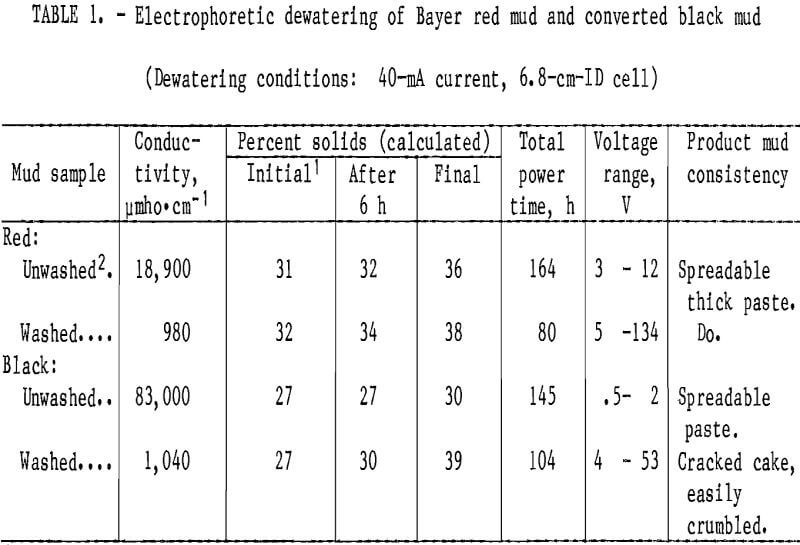
Table 1 summarizes the results obtained from the preliminary electrodewatering experiments on red mud and converted black mud. Muds washed to a conductivity of about 1,000 µmho·cm-¹ dewatered quicker than the unwashed muds, and the dewatered converted black mud was an easier to handle material than the dewatered red mud. Electrodewatering increased the solids content of washed black mud from 27 to 39 pct. Although washed red mud dewatered to approximately the same extent, the black mud appeared more solid. The black mud filter cake cracked and the mud was easily crumbled into small chunks that dried to hard particles after 30 h at ambient conditions. Red mud containing 38 pct solids was a spreadable, thick paste. The unwashed black mud thickened slightly to a gooey paste containing 30 pct solids. These results suggest that the caustic- ferrous digestion and subsequent washing stages caused destabilization of the mud colloid and made particle agglomeration easier. Alteration of the properties of the mud so that the material can be handled by mechanized equipment could eliminate the need for disposal in settling ponds.
Chemical analysis and conductance measurements of the muds and filtrates before and after electrodewatering showed that the sodium and aluminum ions in the mud did not pass out of the cell with the filtrate but concentrated in the dewatered mud. In the case of the washed mud samples, the pH values of the filtrates were several units lower than those of the mud products. Acid produced by anodic oxidation at the lower iron electrode accounts for this decrease in pH (reaction C).
Electrodewatering of Red Mud and Black Muds
from Simulated Bayer Digestion of Bauxite
Electrophoretic dewatering experiments were conducted on black mud samples prepared by adding different amounts of ferrous sulfate to simulated Bayer digestions of Jamaican bauxite. Table 2 shows that production of black mud did not interfere with aluminum extraction except at the highest ferrous concentration.
Table 3 summarizes the results of the electrodewatering tests. The digested muds, which exhibited conductivities
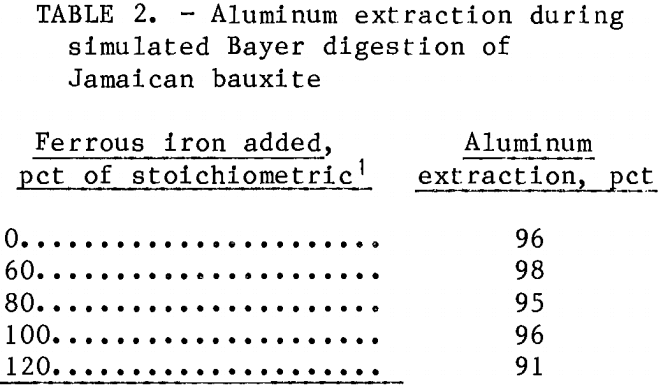
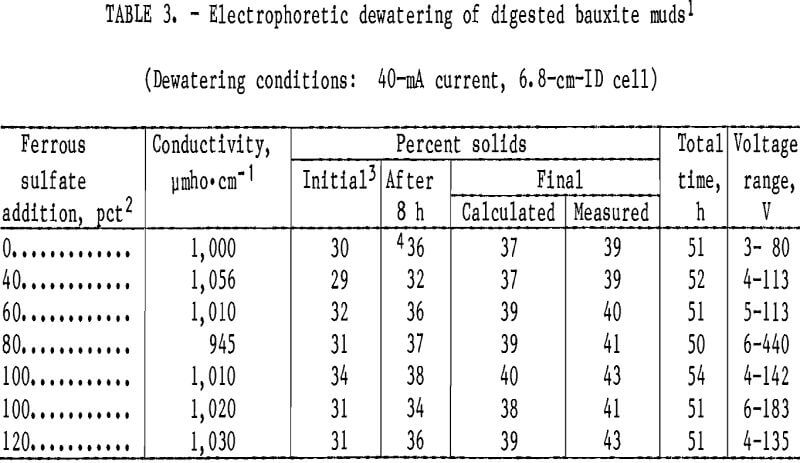
of approximately 115,000 ymho·cm-1, were washed to approximately 1,000 ymho·cm-1 before testing. There was no correlation between the amount of ferrous sulfate added to the digestion step and the final concentration of solids in the mud after electrodewatering. However, red mud (o pct FeSO4) dewatered more slowly than the black muds (fig. 4). This was the result of slower percolation of liquid through the mud bed into the collection flask. Electrophoretic sedimentation of red mud solids occurred during the first few hours after power was applied, separate solid and liquid layers formed, and about 30 h was required to drain the water that accumulated on top of the solid layer. Sedimentation did not occur during black mud testing and most of the dewatering took place during the first 8 h of power application. After the initial fast dewatering, the rate of water loss decreased during the remainder of each experiment and tests were terminated when water stopped flowing into the collection vessel. All of the dewatered muds exhibited pastelike consistencies.
The final solids content could be increased by lengthening the residence time in the test cell, but after the initial fast dewatering period, most of the additional dewatering was due to evaporation, a nonproductive use of energy. This evaporation occurred primarily because the cell voltage increased as the electrodewatering test proceeded and resistive heating of the mud slurry resulted. Even though tests were terminated after only 50 h of power application, evaporation accounted for as much as 50 pct of the weight lost during the power phase of the experiments. A small fraction of the water loss can be attributed to reduction of water and evolution of hydrogen gas at the cathode.
At the end of each experiment, the measured solids content of the mud was consistently higher than the value calculated from equation 1. This can be attributed, at least partially, to the precipitation of iron oxides caused by oxidation of the anode, which in effect adds to the solids in the mud (reaction C). Faraday’s law predicts the quantity of iron corroded from the anode will increase with time. The longer the cell was operated, the greater the observed difference between calculated and measured solids concentration.
The factor that had the greatest effect on the final solids content of a black mud slurry was the solids concentration in the mud after settling and gravity draining. This initial percent solids increased with the length of time the slurry was aged after digestion and is possibly the result of magnetic flocculation of the mud particles. Table 4 shows that aging a black mud sample containing 28 pct solids for 6 to 34 days prior to
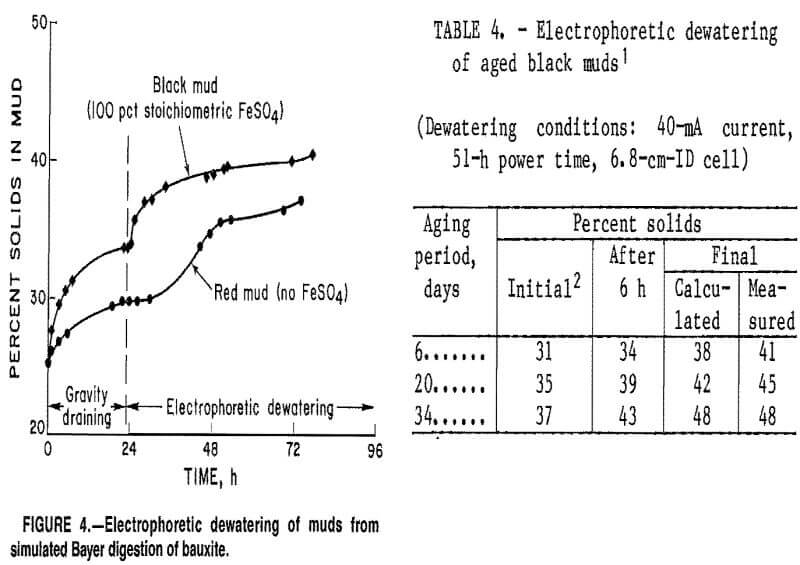
electrodewatering caused the solids content after gravity draining to increase from 31 to 37 pct and the final solids content to increase from 41 to 48 pct. Since Bayer muds are discharged into large settling ponds, aging could be allowed to occur before power was applied to electrodes imbedded in the mud if this dewatering technique were used on an industrial scale.
Tests were performed in a larger cell using converted black mud and red mud from the alumina plant. The muds were washed to 1,000 µmho·cm-1 before testing. The cell had a 9.3-cm ID and a 600-g capacity, compared with the 6. 8-cm ID and 200-g capacity of the smaller cell used in previous tests. It was operated at the same current density as the smaller cell, 1.1 mA/cm². Because of the larger cross-sectional area of this cell, the overall current was also larger (80 mA compared with 40 mA for the 6.8-cm-ID cell), which resulted in higher voltages across the cell. Voltages exceeded 400 V and mud temperatures exceeded 50° C during later stages of the tests.
Electrophoretic sedimentation of the solids occurred when power was applied to the cell and percolation of liquid through the mud bed was slower than the settling rate during the first 3 to 6 h. As a result, separate liquid and mud layers formed in the cell. Eventually the liquid percolated through the mud into the collection flask. By the time the converted mud had dewatered to approximately 32 pct solids, the cake had cracked. Similar results had been observed when converted mud was tested in the 6. 8-cm-ID cell. The converted black mud cake contained 44 pct solids and was crumbly in texture, while red mud dewatered to a thick paste like consistency at final solids content of 41 pct. Evaporation accounted for about 40 pct of the weight lost by the muds during power application.
Summary and Conclusions
Several observations and conclusions can be drawn from the research conducted on electrodewatering of Bayer muds.
- Washed mud dewaters quicker and more extensively than unwashed mud. Addition of more washing stages to make electrodewatering possible could increase processing costs considerably because the muds settle slowly and are difficult to wash.
- Good electrical contact between the thixotropic mud and the electrodes is difficult to achieve as the mud thickens because gassing, especially at the cathode, creates a vapor barrier between the mud and electrode surface. As a result, the voltage drop across the cell increases and resistive heating of the mud occurs, which results in an inefficient use of energy. Large-scale application of electrodewatering would necessitate an electrode design that provides better contact with the mud than was achieved with the test cells. Facilities for venting the offgases also would be required.
- Washed, converted black mud produces an easier to handle dewatered product than washed red mud or black mud formed during simulated Bayer digestion of bauxite in the presence of ferrous sulfate. Alteration of the properties of the mud so that the material can be handled by mechanized equipment could eliminate the need for disposal in settling ponds. However, this advantage would be offset by the expense of a second digestion step to convert the red mud to black mud.
This research has shown on a laboratory scale that electrokinetic densification can be used to enhance the consolidation rate of Bayer mid products. The solids content of presettled muds can be increased from 25 pct to approximately 40 to 48 pct by gravity draining followed by electrodewatering for approximately 48 h. Black muds produced by adding FeSO4 to a simulated Bayer digestion of bauxite or by caustic digestion of red mud in the presence of FeSO4 dewater faster than normal red mud. However, electrodewatering may not be practical because of the increased reagent and processing costs and because the muds must be washed prior to electrodewatering to remove dissolved ions and decrease mud conductivities.
