Wolframite, sp. gr. 7.1 to 7.5, tungstate of iron and manganese, is feebly magnetic; specimens from some localities are reported to be strongly magnetic. Wolframite frequently accompanies cassiterite in tin ores, and on account of their similar specific gravities (cassiterite 6.4 to 7.02) these minerals may not be separated from each other by specific-gravity methods. The method usually followed in treating crude ore or concentrate of this class is to convert any iron, or copper-iron, sulphides which may be present into magnetic compounds by roasting, and separate these from the mixture by low-intensity magnets, then to pass the remainder through a stronger field which takes out the wolframite as a magnetic product and leaves a nonmagnetic tailing carrying the tin. Numerous tests on large quantities of tin-tungsten concentrates made preliminary to the installation of separation plants have yielded high extractions, and a number of these plants are at present in operation in Europe and elsewhere. Most of these installations treat the product of preliminary water concentration, but at least one of them is operating on raw ore. Difficulty has been experienced in the separation of wolframite from cassiterite through a tendency of magnetic particles to adhere to the cassiterite, thereby causing it to be drawn into the magnetic product when a strong field is used for separation; this tendency may be overcome by treating the concentrate with sulphuric acid and drying before pasing it to the separator. Arsenopyrite (sp. gr. 5.67 to 6.3) is of frequent occurrence in ores carrying wolframite: upon roasting this mineral the arsenic is driven off and the resulting magnetic oxide of iron separated from the wolframite by low-intensity magnets. Care must be taken in the roasting of concentrates carrying cassiterite that the heat does not rise to such a degree as to cause particles of magnetic oxide to become attached to the cassiterite particles, and so cause them to be drawn into the wolframite product.
At Gunnislake Clitters, England, tin-tungsten concentrate is being separated on Humboldt-Wetherill separators. The ore from the mine is crushed in breakers and rolls, sized, and the sands concentrated on tables and the slimes with Luhrig classifiers. The concentrate is roasted in a Bruckner furnace having a capacity of 10 tons daily; this material carries, raw, 12 per cent. sulphur and arsenic, principally the former, and the iron with which these elements are combined is rendered strongly magnetic in the roast. The roasted concentrate, after cooling, is fed to a Humboldt-Wetherill separator which carries 4 amperes on the first magnet and 12 amperes on the second. The first magnet removes the strongly magnetic iron compounds and the second the wolframite, while the tin is contained in the nonmagnetic tailing. The tungsten concentrate and the tin product from the separator are both reconcentrated to eliminate waste. The raw ore yields 0.378 per cent. tin and 0.72 per cent. tungsten. The tungsten concentrate carries from 60 to 64 per cent. tungstate of iron, equal to 46 to 49 per cent. WO8. The separator treats 6 tons of concentrate in 10 hours.
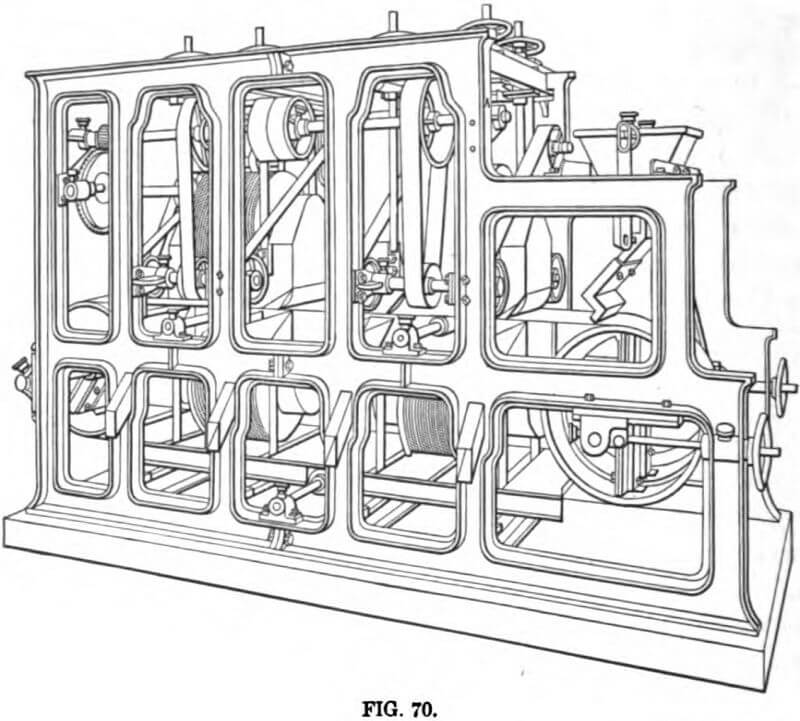
At Redruth, Cornwall, England, the East Pool & Agar United Mines Co. is operating a magnetic-separation plant on concentrates containing wolframite, cassiterite, arsenical pyrites, and chalcopyrite. The ore is crushed by stamps, classified, and con
centrated on Wilfley tables and Frue vanners, which deliver a concentrate carrying cassiterite, wolframite, and arsenical pyrites, the gangue minerals and the chalcopyrite being eliminated by the concentration. These concentrates are roasted and the arsenic driven off, to be recovered in the flues, while the residue is again passed over Wilfley tables, yielding a product consisting of about three parts oxide of tin to one part wolframite, and carrying about 5 per cent. magnetic oxide of iron. After drying, this product is passed over a Humboldt-Wetherill separator, which delivers three products: magnetic oxide taken out by the first and weaker magnet, wolframite taken out by the second and stronger magnet, and a nonmagnetic product carrying the tin.
