Table of Contents
The Stillwater Complex in Montana (mainly the Benbow and Mouat deposits) contains an estimated 15.7 million short tons of ore containing 1.3 million short tons of recoverable chromium. This represents approximately 75 percent of available domestic chromite and could supply U.S. needs for about 4 to 5 years, but it is low in grade and has a low chrome-to-iron ratio.
Currently all U.S. needs for chromium are supplied by foreign imports. Interruptions in supplies of foreign chromite ore are a definite possibility, especially in times of national emergency.
As part of the Bureau of Mines mission to improve mineral technology and thus reduce the Nation’s dependence on foreign imports, research was performed to devise and improve methods for beneficiating domestic chromium ores. This report presents experimental data on amine flotation of chromium ores from the Stillwater Complex, Mont., and gravity concentrates prepared from these ores.
A great deal of beneficiation work on domestic chromite ores has been done by the Bureau of mines. Ores from the Stillwater Complex in Montana have received considerable attention. Gravity separation and froth flotation constituted the majority of the earlier mineral beneficiation work.
The Bureau of Mines, U.S. Department of the Interior, devised a flotation technique for upgrading chromite in chromium ores and gravity concentrates. The technique consisted of cationic flotation of chromite from a slime-free pulp acidified to pH 2.5 or lower using H2SO4 modifier.
Flotation results showed that ore containing serpentine as the primary gangue mineral was more amenable to concentration by this technique than ore containing the readily floatable olivine as the primary gangue mineral.
Rougher flotation of a chromite ore containing abundant serpertine increased the grade form 19 to 41 percent Cr2O3 at a chromite recovery of 89.6 percent. Rougher flotation of ore containing abundant olivine upgraded the chromite from 23.4 to 29.6 percent Cr2O3 at a recovery of 89.4 percent.
The technique was also applied to chromite gravity concentrates to further upgrade chromite and to reject silicates. Flotation of a gravity concentrate containing 40.9 percent Cr2O3 and 4.4 percent SiO2 recovered 90 percent of the chromite in cleaner products containing 44.9 percent Cr2O3, and 0.63 percent SiO2. The combined recovery by gravity and flotation was 87 percent. An economic evaluation of this technique will be undertaken to determine its feasibility for commercial use.
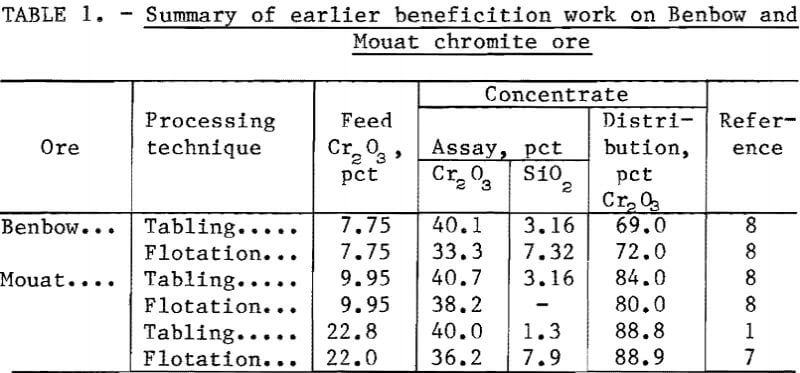
The general flotation method was developed by Havens. Deslimed chromite ore was floated at pH 1.5 to 5.5 using a long-chain fatty-acid collector and soluble fluoride ions as a silicate depressant. The Havens method was later modified slightly to permit flotation without preliminary desliming. This was done by preconditioning the pulp with fuel oil and fluoride ion. The chromite collector was a mixture of a long-chain fatty acid and petroleum sulfonate. The modified Havens method usually resulted in higher chromite recoveries, but with a corresponding reduction in grade. Results of the earlier beneficiation work on Benbow and Mouat chromite ores are presented in table 1.
EQUIPMENT
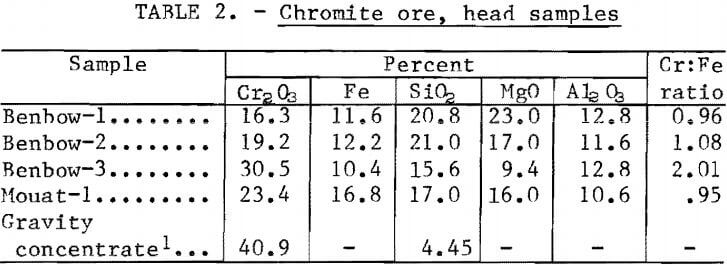
Chromium ore samples for the current beneficiation testing were obtained from the Benbow and Mouat mines in the Stillwater Complex near Nye, Mont. Partial chemical analyses of the head samples are listed in table 2.
Petrographic analyses of the samples showed that the most abundant silicate minerals present in the Benbow ore were serpentine and enstatite. Subordinate amounts of diopside and olivine and small amounts of calcite were also present in this ore. The most abundant silicate minerals in the Mouat ore were olivene and enstatite with serpentine occurring in subordinate amounts and diopside, chlorite, magnetite, labradorite, and biotite occurring in small quantities. Chromite mineral liberation in all ore samples was essentially complete at sizes finer than 65 mesh.
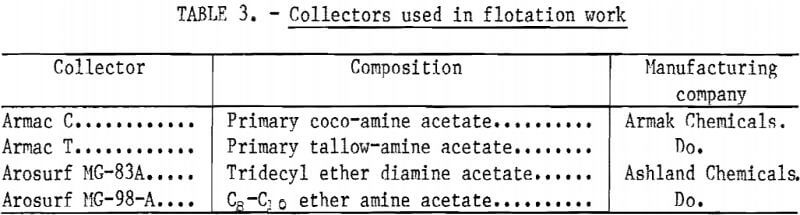
The collectors used in flotation testing are listed in table 3.
Flotation of Chromite Procedure
Bench-scale flotation tests were performed on chromium ores using a Denver laboratory flotation machine. Locked-cycle flotation tests were performed on the gravity concentrate using both the Denver laboratory machine and a larger Denver No. 8 machine. The latter machine was used to prepare large samples of low-silica flotation concentrate for refractory testing.
Unless otherwise noted the general bench-scale flotation procedure consisted of the following steps:
- Grinding 500- to 1,000-gram charges of minus 10-mesh ore at 60 percent solids in a laboratory ball mill. All tests were batch ground for a set period of time.
- Desliming at 5 to 30 micrometers in a 4,000-milliliter beaker by sedimentation-decantation according to Stoke’s law. Sodium silicate was used for dispersion in amounts up to 4 pounds of reagent per ton of ore.
- Pulping the sands to 24 to 29 percent solids in a laboratory flotation cell.
- Adjusting the pH with either H2SO4 or NaOH, monitoring the pH, and adding pH modifier as needed.
- Adding an amine collector as a water-soluble acetate salt and conditioning for 1 minute.
- Floating chromite in three rougher stages where collector is added before each stage. Constant pH was maintained during flotation.
- Cleaning the rougher concentrate. No reagents were added to cleaner flotation except pH modifier.
The gravity concentrate for flotation testing was prepared from Benbow-2 ore using a combined Humphrey’s spiral-tabling technique. The general procedure consisted of a two-stage spiral separation of minus 14-mesh ore, two-stage tabling of the fine and coarse spiral tailings, and blending of the spiral and table concentrates. The two-stage spiral or the table separations involved a rougher and cleaner stage. Coarse tailings sizes were about 14 mesh by 200 mesh and fine tailings were minus 200 mesh.
The procedure for locked-cycle flotation of gravity concentrates in 50-pound batches consisted of—
- Grinding each 50-pound sample through 100 percent minus 100 mesh at 60 percent solids in an 18-inch by 18-inch ball mill.
- Without desliming, pulping to 28 percent solids in a Denver No. 8 flotation cell.
- Conditioning with H2SO4 at pH 2.0 for 10 minutes.
- Conditioning with Armac C for 1 minute followed by a 10- to 15-minute rougher flotation.
- Conditioning again with Armac C for 1 minute followed by a second 10- to 15-minute rougher flotation.
- Combining cleaner tailings with new rougher feed and repeating steps three to six.
The smaller scale locked-cycle flotation procedure was similar to the preceding method, except that 300-gram charges were used.
Results
The effects of pH, type of acid used to modify the pH of the pulp, collector type, preconditioning time with acid, deslime size, and type of ore on flotation grade and recovery were studied using either Benbow or Mouat ores. The flotation technique was further tested on gravity concentrates prepared from Benbow ore.
Effect of Pulp pH on Flotation of Chromite
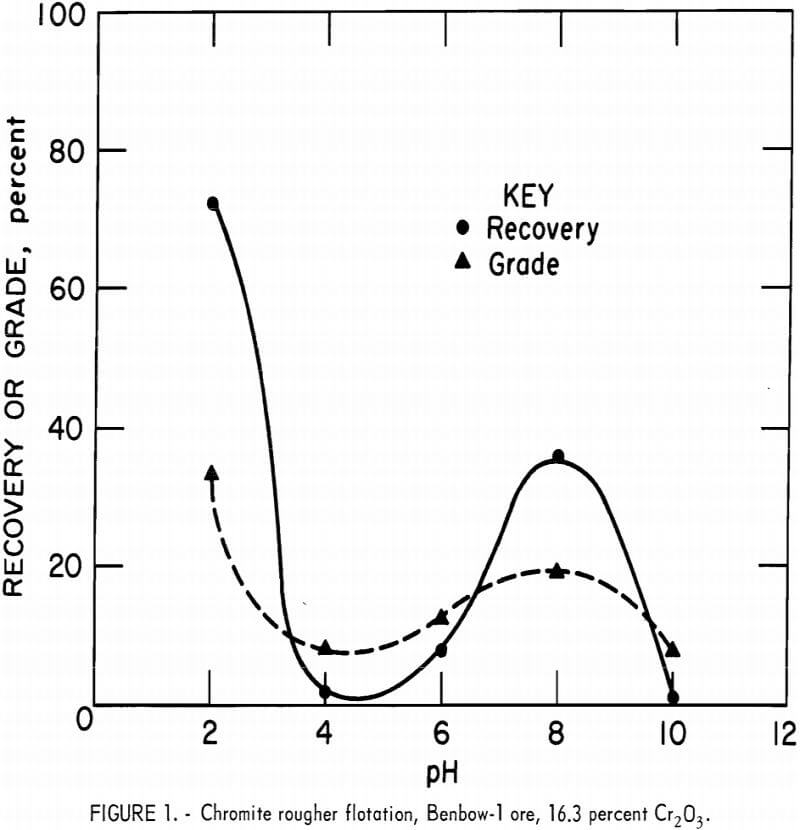
The effect of pH on flotation response was originally performed on Benbow-1 ore (16.3 percent Cr2O3). The ore was ground to 88 percent minus 65 mesh and deslimed at a 30-micrometer particle size. The chromite was floated in three rougher stages with a total of 0.6 pound Arosurf MG-83A per ton of ore.
Figure 1 illustrates flotation response as a function of pH. One small maximum occurred at approximately pH 8 where 35.8 percent recovery was obtained; however, the grade was only 18.7 percent Cr2O3. Maximum flotation occurred at pH 2.0 where a recovery of 73.2 percent was obtained at a grade of 34.0 percent Cr2O3. To obtain a pH of 2.0, H2SO4 consumption was 28.0 pounds acid per ton of ore. Flotation at pH values less than 2.0 was not investigated because of the impractically high H2SO4 concentrations required for pH adjustment.
The point of zero charge for Stillwater chromites generally occurs in the range of pH 5 to 7. If the pulp is more basic than pH 7, the chromite surface exhibits a net negative charge. If the pulp is more acidic than pH 5, the chromite surface exhibits a net positive charge. Collector adsorption at pH 8 may be attributed to electrostatic attraction between the positive amine and the negative chromite surface. Collector adsorption and thus chromite flotation at pH 2 is probably due to sulfate ion from the H2SO4 acting as a bridge between the positive amine and positive chromite surface.
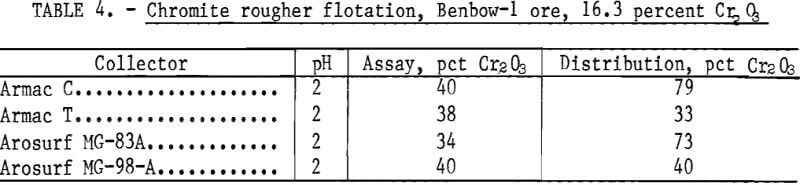
Effect of Collectors on Flotation of Chromite Ore
Four different amine collectors were investigated using the 16.3- percent-Cr2O3 Benbow-1 ore. The flotation procedure was as described previously. For each test, collector dosage was 0.6 pound per ton and H2SO4 consumption was 26 pounds per ton. Rougher flotation using Armac C yielded 79 percent recovery in a 40 percent Cr2O3 product. Results presented in table 4 show that Armac C, a primary coco-amine, was the best reagent.
The collector quantity used and reported for each ore sample was established by preliminary tests where collector concentration was varied. With the 16.3-percent-Cr2O3 Benbow-1 ore, varying the Armac C addition from 0.1 to 1.0 pound per ton showed that optimum chromite flotation occurred with 0.6 pound Armac C per ton of ore.
Effect of pH Modifier Flotation of Chromite
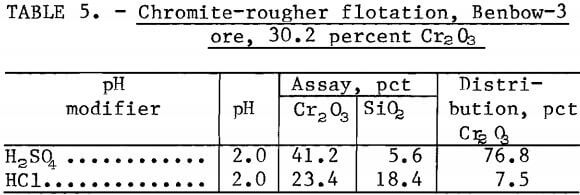
Identical flotation tests were done on samples of Benbow-3 ore containing 30.2 percent Cr2O3 using H2SO4 or HCl as pH modifier. The samples were ground to 100 percent minus 100 mesh, deslimed at a 30-micrometer particle size, and floated with 0.6 pound Armac C per ton of ore. Table 5 shows that when H2SO4 was the pH modifier, 76.8 percent recovery was obtained at a grade of 41.2 percent Cr2O3 and 5.6 percent SiO2. When HCl was the pH modifier, a recovery of only 7.5 percent was obtained at a grade of 23.4 percent Cr2O3 . Sulfuric acid clearly has a beneficial effect on chromite flotation with an acidic pH.
Preconditioning Acid Scrub
Acid scrubbing as a preconditioning step was investigated with 19.2- percent-Cr2O3 Benbow-2 ore. The ore was ground to 100 percent minus 100 mesh and deslimed at a 30-micrometer particle size. The flotation procedure included 0-, 1-, 5-, and 20-minute conditioning periods in the flotation cell with sulfuric acid at pH 2.0. Total reagent consumptions were 1.0 pound of Armac C and 13.0 to 21.0 pounds of H2SO4 per ton of ore. Results (table 6) show that acid scrubbing caused a significant increase in flotation response. Raising the conditioning time from 0 to 20 minutes resulted in a recovery increase from 63.5 to 89.6 percent and a grade increase from 39.6 to 41.0 percent Cr2O3. The majority of this recovery increase occurred in the first 5 minutes of conditioning. To take advantage of the beneficial effect of acid scrubbing while keeping conditioning time to a minimum, a conditioning time of 10 minutes was adopted in subsequent test work. Conditioning times in excess of 10 minutes were found to be only slightly beneficial.
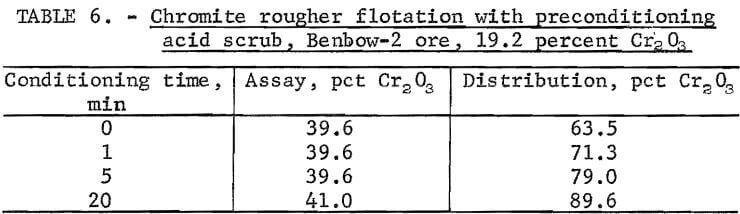
The improvement in chromite grade and recovery with prolonged scrubbing may be due to cleaning of the mineral surfaces. Similar phenomena were observed when purified chromite mineral was acid-washed with hydrochloric acid prior to flotation with either dodecylamine or dodecylsulfate collector.
Effect of Desliming
A series of tests was performed on Benbow-2 ore (1) to determine the effect of desliming size on chromite grade and recovery in the rougher concentrate and (2) to determine chromite losses in the slimes. Each sample was ground to 100 percent minus 100 mesh, deslimed at the appropriate size, and conditioned at pH 2 for 10 minutes with H2SO4; the chromite was then floated with 0.6 pound Armac C per ton of ore. The results (table 7) show that as the desliming size increased from 5 to 20 micrometers, only small changes in concentrate grade and recovery occurred. The chromite grade varied from 39.6 to 41.0 percent Cr2O3 and recovery varied from 89.7 to 85.3 percent. The optimum desliming size was 10 micrometers, where flotation of the sands resulted in 89.6 percent chromite recovery in a 41.0-percent Cr2O3 grade concentrate. With no desliming, the grade was 32.6 percent Cr2O3 and the recovery was 23.0 percent. This poor flotation response was attributed to adsorption of collector on the slimes and the formation of a slime coating on the chromite particles.
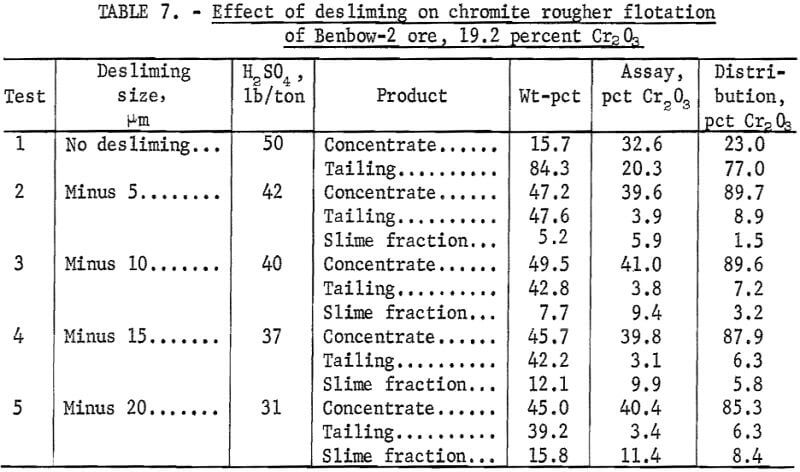
Loss of chromite values to the slime fractions ranged from 1.5 to 8.4 percent as the desliming size increased. Although desliming at 30 micrometers was not included in this series of tests, other test results indicate about 10 percent of the chromite is lost to the 30-micrometer slime fraction.
Sulfuric acid consumption increased from 31 pounds H2SO4 per ton of ore with a 20-micrometer desliming size to 50 pounds H2SO4 per ton of ore when there was no desliming. The sulfuric acid requirement increased significantly as the percentage of slimes in the flotation pulp increased.
Effect of Ore Type
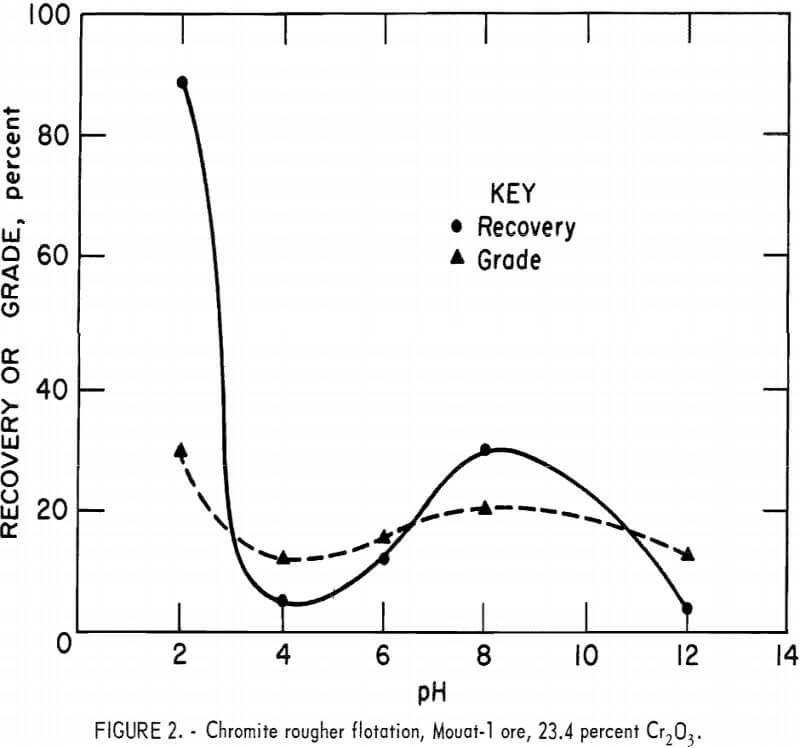
Amine flotation research was also performed on an ore sample from the Mouat mine. The ore sample contained 23.4 percent Cr2O3. It was ground to 100 percent minus 65 mesh, deslimed at the 20-micrometer particle size, and floated in a three-stage rougher using a total of 0.4 pound of Armac C per ton of ore.
Figure 2 illustrates flotation response as a function of pH. The shape of the curve is the same as the one obtained from flotation of Benbow ore (fig. 1), Maximum flotation again occurred at pH 2.0 where 89.4 percent recovery was obtained at a grade of 29.6 percent Cr2O3. Although recovery was very high, the selectivity was poor at pH 2.0. The grade of product did not increase significantly in cleaner flotation. Conventional silicate depressants had no effect on selectivity.
Olivine is the predominant silicate mineral in the Mouat ore. Petrographic examination of the pH 2.0 flotation products showed olivine to be present in the concentrate while serpentine remained in the tailings. To increase the selectivity of Mouat ore to amine flotation of chromite, a selective olivine depressant must be found.
Flotation of Chromite Gravity Concentrate
The amine flotation method was applied to chromite gravity concentrates prepared from Benbow-2 ore by a combined Humphrey’s spiral and table separation technique. The purpose of re-treating the gravity concentrate was to prepare a low-silica feed material for refractory testing.
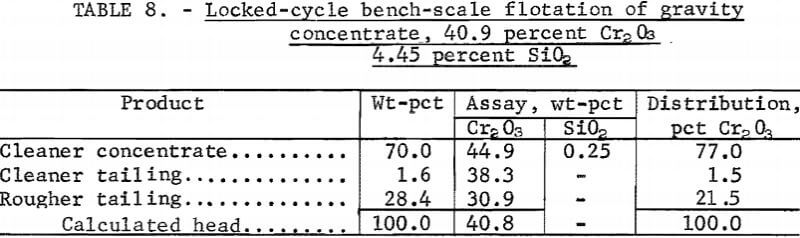
Preliminary locked-cycle bench-scale tests were performed on a gravity product containing 40.9 percent Cr2O3 and 4.45 percent SiO2 using a flotation procedure similar to the one described for the large-scale locked-cycle tests. Results (table 8) show that five cycle operations produced flotation cleaner concentrate containing 45 percent Cr2O3 and 0.25 percent SiO2 at a chromite recovery of 77.0 percent. Reagent consumptions were 0.5 pound of Armac C and 40 to 50 pounds of H2SO4 per ton of feed.
About 500 pounds of low-silica concentrate was prepared by locked-cycle flotation in the Denver No. 8 cell using 50-pound charges of gravity concentrate. Reagent consumptions were 0.6 pound of Armac C and 50 pounds of H2SO4 per ton of feed.
The results (table 9) are weighted averages of the locked-cycle flotation tests and show a 90.4 percent chromite recovery in a cleaner concentrate containing 44.8 percent Cr2O3 and 0.6 percent SiO2. Concentrates of this grade were obtained consistently in all cleaner flotation froths; the chromite grade ranged from 44.5 to 45.0 percent and the SiO2 content ranged from 0.5 to 0.8 percent.
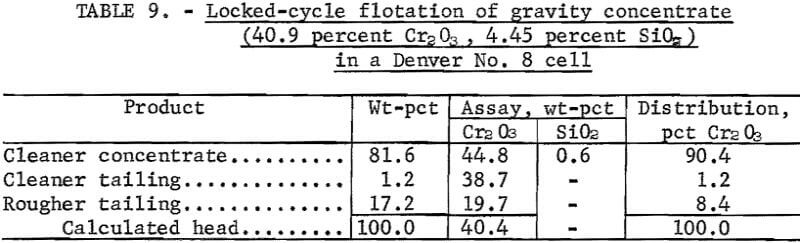
Sulfuric acid consumption for pH adjustment was over twice as high as consumption in the direct flotation of deslimed ore. Because the gravity concentrate flotation feed was not deslimed, the higher acid consumption is attributed to the presence of finer sized particles after ball milling of the minus 14-mesh gravity concentrates. The increase of finer sized particles increases the dissolution rate of the minerals and the adsorption of H2SO4. Dissolution of magnesium silicate minerals could generate soluble magnesium sulfate. Through 12 locked-cycle repetitions, neither soluble magnesium sulfate nor any other dissolution species had any effect on acid consumption or chromite flotation.
Conclusions
Experimental results showed that chromite can be readily floated with a cationic collector from an acidic, slime-free pulp. Maximum grades and recoveries occurred at pH 2.0 using from 20 to 50 pounds per ton H2SO4 as a pH modifier and 0.4 to 1.0 pound per ton of Armac C, a primary amine collector. Hydrochloric acid was ineffective as a pH modifier. Precondition scrubbing with H2SO4 at the pH of flotation reduced collector consumption and improved flotation response. Chromite losses in the slime varied from 1.5 to 10 percent depending on the desliming size.
The Benbow ore was more amenable than the Mouat ore to the flotation technique. The difference was not in chromite flotation but in the degree of flotation of siliceous gangue. For example, olivine, the main gangue mineral of Mouat ore, floated with the amine collector, whereas serpentine, the main gangue mineral in Benbow ore, did not float.
The amine flotation technique was very effective in producing a low-silica concentrate from a gravity concentrate. However, sulfuric acid consumption was high, because of the, presence of fine particles generated during regrinding of the gravity product prior to flotation. An evaluation of this technique will be performed to determine its economic feasibility for commercial use.
